1. 引言
PLLA和PLGA具有优良的生物相容性和可降解性,降解产物是二氧化碳和水,降解产物不会在体内聚积,降解速率可通过相对分子量控制,已广泛的用作细胞支架材料[1,2]。羟基磷灰石一直被认为是骨组织中重要的无机成分,能够诱导细胞增殖和分化[3],同时能够增强材料的机械强度。如果将羟基磷灰石与PLGA进行复合,既能提高支架材料的机械强度,又能增加材料的骨诱导性,且对PLGA的降解速度有控制作用,以保证材料的降解速度与骨组织的再生速度一致[4]。
骨再生是一个涉及到多种细胞因子作用的复杂过程[5]。骨是一个高度血管化的组织,血管生成对骨再生至关重要[6]。研究表明,骨修复过程中血管生成早于骨生成[7-11],构建的骨修复体应首先诱导血管生成,再诱导骨生成,即先释放诱导血管生成的细胞因子,再释放诱导骨生成的细胞因子。而现常用的方法以各种材料的微球或支架为缓释载体,未考虑生长因子作用的时间依赖性。若将微球与支架复合,将不同生长因子分别载入微球和支架,使不同的生长因子达到不同的释放规律,有望更好的实现骨修复。
传统制备多孔支架材料的方法有相分离法、纤维粘接及溶液浇铸–粒子沥滤法,但涉及到有机溶剂或较高的温度,会破坏生物分子的活性,突释现象也较为严重。超临界流体技术无污染、无高温、产品无有机溶剂残留、绿色环保,能将蛋白载入支架材料或微球内而不失去生物活性、对支架材料的力学性能和内部孔型有良好的控制[12-16]。
实验以胰蛋白酶(Try)为模型蛋白,采用复乳法制备Try-PLLAms,复合nHA/PLGA后经超临界流体发泡形成Try-PLLAms/nHA/PLGA复合支架。考察其蛋白缓释行为,为构建具有生长因子次第释放效果的组织工程支架奠定实验基础。
2. 材料和方法
2.1. 材料
PLLA (MW = 10,000) (山东医疗器械研究所),PLGA (山东医疗器械研究所),nHA (国药集团化学试剂有限公司),Try (美国Sigma公司),BCA试剂盒(南京建成生物工程研究所)。
2.2. Try-PLLAms的制备
称取250 mg PLLA溶于5 mL二氯甲烷中组成有机相,待其完全溶解后,加入250 μL 1 mg/mL的Try溶液,至于磁力搅拌器上搅拌2 min,再加入10 mL 1% PVA磁力搅拌5 min形成乳浊液。将乳浊液加入400 mL 0.1% PVA中搅拌2 h,有机溶剂挥发后得到微粒的混悬液。将所得微粒的混悬液5000 r/min离心10 min,沉淀用蒸馏水洗涤3次,真空冷冻干燥24 h得到PLLAms。
2.3. Try-PLLAms/nHA/PLGA复合支架的制备
将PLGA与nHA按一定的比例研磨,研磨时间为4 h,加入10%的PLLAms,将混合粉末放入直径为5 mm的圆柱形模具中进行压制,置于四氟乙烯圆柱体模具内并将模具放入容积为500 mL的超临界CO2不锈钢反应釜中,密封后用液泵将冷凝至0℃以下的CO2压入反应釜内,当反应釜内压力达到8 MPa时开始升温,使反应釜内温度达到35℃,通过仪器内置的热电偶和放空阀调节釜内的压力和温度,保持温度变化不超过±0.5℃,压力变化为±0.1 MPa。反应8 h后,以一定的速率放气,打开反应釜,取出样品,按以上方法,不加入PLLAms,直接加入5 mg Try,制备TrynHA/PLGA支架。
2.4. PLLAms和复合支架形态观察
将空白和含Try的PLLAms分散到导电胶上,喷金,观察微球的表面形貌。取数块制备的复合支架,喷金后观察(JSM-5900LV,Japan)支架内部结构形态。
2.5. 微球载药量和包封率测定
称取50 mg PLLAms溶解在10 mL二次水中,样品中附着在微球外的蛋白会溶解在二次水中,经抽滤后,将微粒溶解在5 mL二氯甲烷中,再加入40 mL二次水,经磁力搅拌使二氯甲烷溶剂挥发完毕,BSA蛋白即被溶解在水中,然后对蛋白溶液用0.22 μm的水膜过滤,收集滤液,按照BCA试剂盒测定蛋白含量。载药量和包封率的公式分别为:
载药量 = 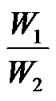 × 100%,包封率 =
× 100%,包封率 = 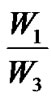 × 100% W1为复合微球内的药量;W2为复合微球的总的质量。W3为复合微球内药量及介质中的药量。
× 100% W1为复合微球内的药量;W2为复合微球的总的质量。W3为复合微球内药量及介质中的药量。
2.6. 支架孔隙率测定
称量样品的干重记为G1,测量样品的长度及半径,分别记为L和R,将样品放入二次水中抽真空,使水充分的渗入到材料中,直到材料表面没有气泡冒出为止。取出样品,用滤纸吸干表面的水分,称重,质量记为G2,那么根据公式,孔隙率为:
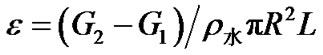
2.7. 支架降解试验
将直径为5 mm,长度为10 mm的样品浸泡于pH 7.4的磷酸缓冲液(PBS)中,抽真空,使样品充分润湿。将完全润湿的样品放入50 mL磨口瓶中,加入一定量的PBS溶液,放入37℃恒温水浴摇床内。分别于时间点0,1,2,3,4,6,8,10,12周取样,去离子水冲洗,冻干后称重,计算质量损失,公式为:
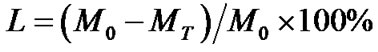
其中,L为质量损失,M0为降解前支架的质量,MT为降解不同时间后支架的质量。
2.8. 蛋白体外释放试验
精密称取一定量的复合支架、Try-PLLAms、TrynHA/PLGA支架,以pH 7.4的PBS 10 mL为释放介质,置于37℃恒温摇床中摇动。每隔一定的时间取出20 μL上清液,并补充等量的PBS,用BCA蛋白检测试剂盒检测蛋白含量,计算累积释放率。
3. 实验结果
3.1. 微球的形态及粒径
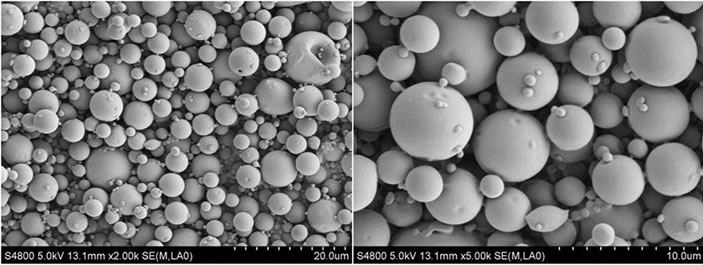
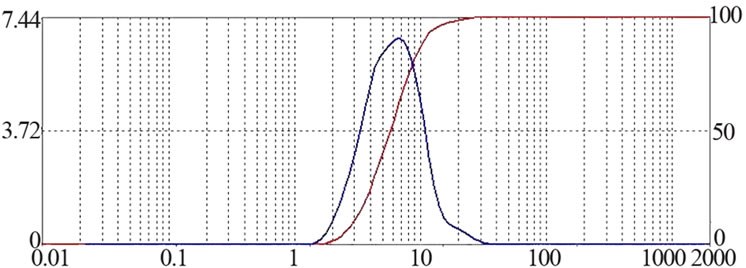
所制备的Try-PLLAms形态良好,呈规则的球形,表面光滑,无粘连现象,但粒径大小不均匀,可能是因为在搅拌过程中转速很难在长时间保持稳定造成的,见图1。所得微球粒径分布较窄,为2~7 μm,成近似正态分布,其中,空白PLLAms的平均粒径是4.8 μm,Try-PLLAms平均粒径是6.1 μm,见图2。
 (a)
(a)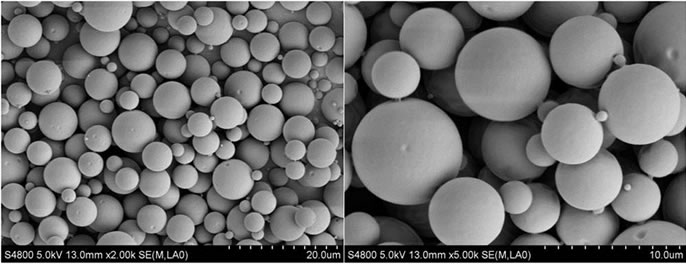 (b)
(b)
Figure 1. SEM micrographs of PLLA microspheres (a): Unloaded microspheres (b): Try loaded microspheres
图1. PLLA微球的SEM图片 (a):空白微球 (b):载Try的微球
 (a)
(a)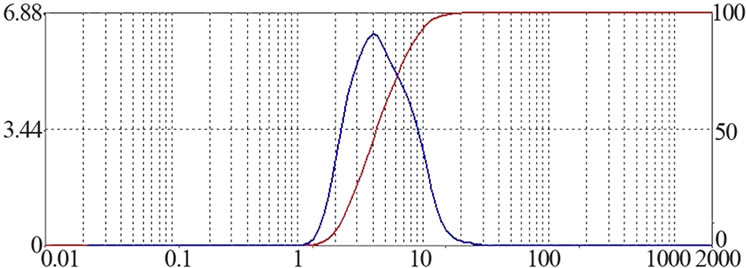 (b)
(b)
Figure 2. Size Distribution of PLLAms (a): Unloaded microspheres (b): Try loaded microspheres
图2. PLLA微球的粒径分布 (a):空白微球 (b):载Try的微球
3.2. 复合支架的形态、孔隙率和抗压强度
制备的复合支架孔径分布在150~300 μm,孔连通性较好,具有较高的孔隙率,PLLAms均匀的分布在支架上,见图3,箭头所指为PLLAms。复合支架孔隙率50.9%~76.8%,抗压强度3.9~5.1 MPa。不同分子量的PLGA孔隙率和抗压强度见表1。
3.3. 聚乳酸微球的载药量和包封率
使用BCA蛋白检测试剂盒测定PLLAms的载药量和包封率,载药量为0.89%,包封率为80.5%,表明所制备的微球具有良好的载药性能。
3.4. 复合支架的降解和蛋白的释放
随着降解时间的增加,支架材料的质量逐渐减小。降解到第2,4,6,8,10周时,复合支架的质量分别减少3.6%,8.3%,13.5%,19.8%,27.5%。TrynHA/PLGA支架、PLLAms和复合支架在前2天都有突释现象,但复合支架的释放速率整体上比前两者慢,见图4。Try-nHA/PLGA支架,Try 48 h累积释放量达85%以上;PLLAms,Try 48 h累积释放率为65.2%;复合支架,Try 48 h累积释放量为32.9%,21 d累积释放量为60.6%。
4. 讨论
4.1. PLGA分子量对支架性质的影响
表1比较了不同分子量的PLGA的孔隙率和抗压

Figure 3. SEM micrographs of composite scaffolds
图3. 复合支架形态的扫描电镜观察
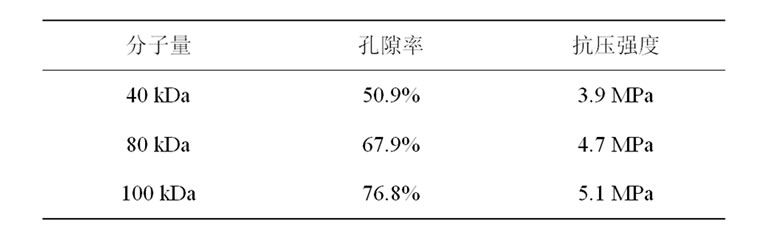
Table 1. Effect of PLGA molecular weight on scaffold properties
表1. 不同分子量的PLGA制得的支架材料的性质
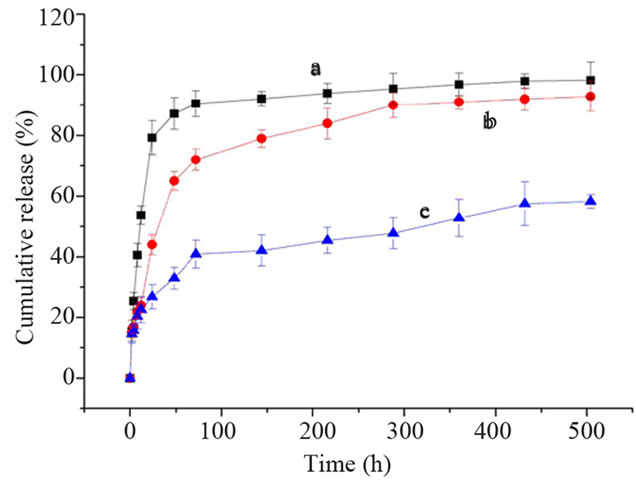
Figure 4. Cumulative release of Try (a) Try-nHA/PLGA scaffold; (b) Try-PLLAms; (c) Try-PLLAms-nHA/PLGA scaffold)
图4. T不同体系体外蛋白释放效果比较 (a) Try-nHA/PLGA 支架;(b) Try-PLLAms;(c) Try-PLLAms-nHA/PLGA支架)
强度。随着分子量的增加,PLGA制得的支架的孔隙率和抗压强度均得到增加。这可能是因为较大分子量的PLGA使聚合物的结晶性降低,有利于超临界CO2的溶胀和气泡核的生长,形成更多的孔洞结构,所以分子量增大孔隙率也增大。而当分子量较大时,聚合物的分子链更长,链段间的有序性降低,使得聚合物链段间的反应更为激烈,聚合物链段之间相互缠绕使它们的力学强度得到增强,从而导致抗压强度的增强。而事实上,孔隙率的增大会导致抗压强度的降低,所以此结论仅适用于特定的范围内。
4.2. 超临界发泡过程对孔隙率的影响
超临界CO2 (TC = 31.1℃,PC = 73.8 bar)的密度与液体密度大小相似,具有较强的溶解能力,粘度与气体相似,扩散速率高[17]。CO2溶解到无定形的聚合物后会使聚合物的玻璃化转变温度、粘性、界面张力和渗透性发生改变[18],同时生成多孔结构。超临界CO2发泡过程分为两个阶段:浸润阶段和减压阶段[19]。在浸润阶段,聚合物被超临界CO2所饱和,降低了聚合物的玻璃化转变温度,聚合物变得有弹性。在减压阶段,压强的降低导致了气泡核的形成,气泡核继续生长产生孔。随着压强的减小,CO2的浓度也降低,聚合物的玻璃化转变温度升高,聚合物就定型为多孔的结构。
用于骨修复的支架材料应具有一定的形状、力学强度、合适的孔径、孔隙率和比表面积等。一般认为支架的孔径必须大于100 μm,否则细胞和组织很难长入支架的内部,且支架内部不易得到充足的养料[20]。实验以超临界CO2为发泡剂,避免了以往方法制备支架致孔剂残留问题。对于骨组织工程支架而言,孔隙率应当大于80%,因此,为了进一步提高支架的孔隙率,可以增加超临界CO2的压强,使更多的气体浸入到聚合物中,也可以加快放气速率,浸入到聚合物基质中的CO2能更快地从基质中析出形成更为连通的孔。如果释放速率过低,仅仅造成聚合物基质的溶胀,孔隙率较低。
4.3. 复合载体系统释放规律的应用
载体系统主要功能就是在局部保持药物缓慢释放,以达到药物最大的生物学性能[21]。以Try为模型蛋白制备PLLAms,对Try具有缓释作用,但在组织工程应用时无法塑形,故将PLLAms复合到具有一定形态和机械强度的nHA/PLGA支架上,使其形成符合要求的缓释载体系统,进而得到理想形态的组织工程骨。孔径在150~300 μm nHA/PLGA复合支架,加强了PLLAms对Try的释放,起到二次缓释作用,从而使Try的释放更为缓慢。如果同时将另一种蛋白直接加入nHA/PLGA粉末中,再与载蛋白的PLLAms混合发泡,可能会使不同的蛋白有不同的缓释效果。在骨修复中可以将诱导血管生成的细胞因子直接混入nHA/PLGA粉末中,诱导骨生成的细胞因子载入PLLAms中,将微球与nHA/PLGA粉末混合发泡,可以使支架优先释放诱导血管生成的细胞因子,后释放诱导骨生成的细胞因子,可能会达到更好的骨修复效果。Thomas等[22]制备包裹血小板衍生生长因子的聚丙交酯–乙交酯(PLG)微球,然后复合到混有血管内皮生长因子的PLG支架上,植入到骨缺损处,达到良好的成骨效果。
4.4. 复合支架制备过程对蛋白活性的影响
保持药物活性是实验的重要环节。实验中使用2%冰醋酸溶解Try,不会引起蛋白类药物变性,冰浴机械搅拌2 h,有利于醋酸的充分挥发,使其对药物的影响降到最小。采用的超临界发泡技术也是为了保持Try的活性,从而制备具有良好生物学性能的缓释载体系统。
实验制备的Try-PLLAms/nHA/PLGA复合支架可良好的保持Try的生物活性,对Try具有良好的缓释作用,有望作为组织工程支架和生长因子缓释载体。
NOTES
*资助信息:国家自然科学基金(51173120)。
#通讯作者。