1. 引言
手性多元酰胺醇是重要的精细化工中间体,广泛用于合成手性多噁唑啉[1] -[8] 。手性多元酰胺醇的合成方法已见文献报道,主要有三种方法:(1) 以多元羧酸为原料,经酰化后与手性β-氨基醇反应,合成了手性多元酰胺醇[2] [6] 。该方法副反应多,产率低。(2) 以多元羧酸为原料,与手性β-氨基醇直接反应,合成手性多元酰胺醇。该方法副反应少,后处理简便,产率较高[7] 。(3) 以多元羧酸酯为原料,与手性β-氨基醇直接反应,合成手性多元酰胺醇[1] [6] 。该方法副反应少,产率较高,但要先制备多元羧酸酯,而多元羧酸酯的合成难度较大。上述文献报道方法仅见于手性二至五元酰胺醇的合成,而六元酰胺醇的合成仍未见文献报道。本文采用第二种方法来合成新的手性六元酰胺醇N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺[(R)-1或(S)-1]。以三乙四胺六乙酸为原料,与(R)- 或(S)-2-氨基-1-丁醇作用,一步反应合成了目标物,其合成路线见图1。
并以苯乙酮为反应底物模型,二苯基硅烷为氢硅化试剂,初步研究了新合成的手性配体(R)-1或(S)-1与[Rh(COD)Cl]2在苯乙酮的不对称氢硅化反应中的催化活性(见图2)。
2. 实验方法
2.1. 主要仪器与试剂
Vector 22型FT-IR红外光谱仪;Varian UNITY INOVA-500型核磁共振仪,TMS为内标;LCQ DECA
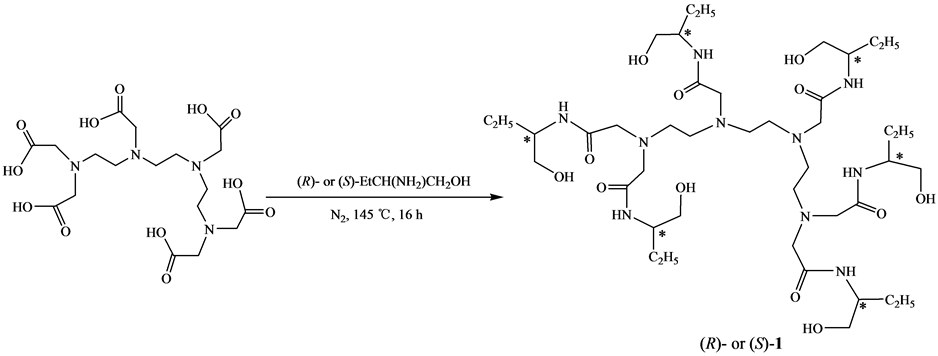
Figure 1. Synthesis of chiral triethylenetramine-N, N, N', N'', N''', N'''-hexa-[2-(1-hydroxybutanyl)]acetoamide
图1. 手性N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺的合成
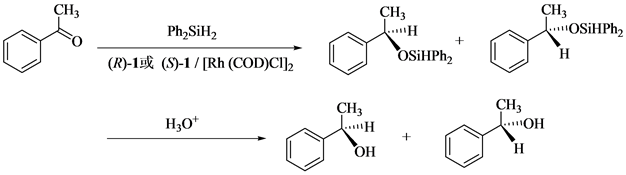
Figure 2. Asymmetric hydrosilylation of acetophenone
图2. 苯乙酮的不对称氢硅化反应
XP液相色谱-质谱联用仪;Polartronic HNQW5旋光仪;Agilent HP-1100 HPLC (手性柱:Daicel Chiralcel OJ-H)。(R)-或(S)-2-氨基-1-丁醇 (Fluka公司);异丙醇和正己烷为市售GC级;其它均为市售AR级试剂。
2.2. 手性N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺[(R)-1或(S)-1]的合成
在50 mL圆底烧瓶中,加入1.00 g (2.02 mmol)三乙四胺六乙酸和1.30 g (14.58 mmol) (R)- 或(S)-2-氨基-1-丁醇,在氮气保护145℃下搅拌16 h。冷却,以乙醇作洗脱剂,用200~300目硅胶进行柱层析,蒸除洗脱剂,得产物(R)-1或(S)-1。
(R)-(+)-N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺[(R)-1]:无色油状粘稠液,产率为69.5%,[α]D25 = +853 (c = 0.13, EtOH);IR (KBr, ν/cm-1):3302,3089,2966,2935,2877,1653,1561,1461,1381,1059;1H-NMR (500 MHz, CD3OD),δ:0.92~0.97 (m, 18H),1.44~1.50 (m, 6H),1.62~1.67 (m, 6H),2.73~2.76 (m, 12H),3.25 (s, 8H),3.28 (s, 4H),3.53~3.60 (m, 12H),3.81~3.85 (m, 6H),其中12个活泼氢被氘代了;ESI-MS,m/z (%):922 ([M+H]+, 100)。
(S)-(-)-N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺[(S)-1]:无色油状粘稠液,产率为71.3%,[α]D25 = −856 (c = 0.15, EtOH);1H-NMR和ESI-MS同(R)-1。
2.3. 苯乙酮的氢硅化反应
手性配体(R)-1[或(S)-1] (0.10 mmol)、[Rh(COD)Cl]2 (0.15 mmol)、苯乙酮(2.0 mmol)和THF (10.0 mL)在氮气保护下室温搅拌1 h,然后在−5℃下加入二苯基硅烷 (3.2 mmol),随后继续在此温度下反应至苯乙酮消失。反应混合液中加入甲醇 (1.0 mL),然后在0℃下加入稀盐酸酸化,分出有机层,水层用乙醚萃取,合并有机层,用无水硫酸镁干燥。蒸除溶剂,残留物以氯仿作洗脱剂,用200~300目硅胶进行柱层析,蒸除洗脱剂,得产物。
(R)-1-苯基乙醇:产率为98%,[α]D25 = +16.2 (c = 5.0 , CH3OH),HPLC分析(手性柱:Daicel Chiralcel OJ-H,流动相:2%异丙醇/正己烷,流速:1.0 mL/min,保留时间:tR (minor) = 10.5 min, tR (major)= 13.0 min)给出异构体的ee值为36%。1H-NMR (300M Hz, CDCl3),δ:1.37 (d, J = 6.79 Hz, 3H),3.05 (br s, 1H),4.76 (q, J = 6.04 Hz, 6.79 Hz, 1H),7.18~7.40 (m, 5H);ESI-MS,m/z (%):123 ([M+H]+, 100)。
(S)-1-苯基乙醇:产率为97%,[α]D25 = −15.8 (c = 5.0, CH3OH),HPLC分析(手性柱:Daicel Chiralcel OJ-H,流动相:5%异丙醇/正己烷,流速:0.5 mL/min,保留时间:tR (major) = 22.0 min,tR (minor)= 25.1 min)给出异构体的ee值为35%。1H-NMR (300M Hz, CDCl3),δ:1.35 (d, J = 6.79 Hz, 3H),3.08 (br s, 1H),4.71 (q, J = 6.04 Hz, 6.79 Hz, 1H),7.15~7.23 (m, 5H);ESI-MS,m/z (%):123 ([M+H]+, 100)。
3. 结果与讨论
3.1. 手性N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺[(R)-1或(S)-1]的合成
手性多元酰胺醇的合成已见文献报道[1] [2] [4] -[7] ,其中以多元羧酸与手性氨基醇直接反应一步合成手性多元酰胺醇的方法比较简便、副反应少和产率高[7] 。但该方法仅见于手性五元或五元以下的酰胺醇的合成。本文采用上述相似的方法来制备新型手性六元酰胺醇。以三乙四胺六乙酸为原料,在氮气保护145℃下与(R)- 或(S)-2-氨基-1-丁醇搅拌16 h,一步反应合成了(R)-(+)-N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺[(R)-1]或(S)-(-)-N, N, N', N'', N''', N'''-六-[2-(1-羟丁基)]三乙四胺六乙酰胺[(S)-1],产率分别为69.5%和71.3%,产物经IR、1H-NMR和ESI-MS谱确证。
3.2. 苯乙酮的不对称氢硅化反应
酮的不对称氢硅化反应已见文献报道[9] [10] 。但使用手性多元酰胺醇作为催化剂进行的酮不对称氢硅化反应仍未见文献报道。本文以苯乙酮为反应底物模型,二苯基硅醚为氢硅化试剂,研究了新制备的手性六元酰胺醇(R)-1或(S)-1与[Rh(COD)Cl]2对苯乙酮的催化氢硅化反应,并考察了溶剂、反应时间、反应温度、配体及其用量等因素对反应对映选择性的影响,结果见表1。
从表1可知:(1) 在苯乙酮的不对称氢硅化反应中,手性六元酰胺醇(R)-1或(S)-1对反应具有较明显的催化作用,但反应的对映选择性较低(ee值最高仅为37%),而产物的绝对构型与形成(R)-1或(S)-1的原

Table 1. Asymmetric hydrosilylation of acetophenone catalyzed by ligand 1 and [Rh(COD)Cl]2
表1. 配体1和[Rh(COD)Cl]2催化苯乙酮的不对称氢硅化反应
a反应条件:[Rh(COD)Cl]2 (0.15 mmol),苯乙酮 (2.0 mmol),二苯基硅烷 (3.2 mmol)和溶剂 (10 mL);b产物的绝对构型和ee值通过HPLC (手性柱:Daicel Chiralcel OJ-H柱)分析决定。
β-氨基醇的构型一致。(2) 影响苯乙酮不对称氢硅化反应对映选择性的因素较多,包括溶剂、反应时间、反应温度、配体及其用量等。对配体(R)-1而言,考虑溶剂效应,反应倾向于使用THF作溶剂为佳。而反应时间、反应温度和配体用量对苯乙酮的氢硅化反应的对映选择性有较大的影响。反应温度升高,反应时间缩短,但产物的ee值下降;配体的用量增加,产物的化学产率增加,产物的ee值增大。
总之,以THF为溶剂,配体(R)-1与[Rh(COD)Cl]2在催化苯乙酮与二苯基硅烷的不对称氢硅化反应中,配体的用量为5 mol% (相对于苯乙酮的量而言),−5℃下反应90 h,产物的化学产率为98%,ee值仅为36%。在相似的反应条件下,配体(S)-1与[Rh(COD)Cl]2对苯乙酮与二苯基硅烷的不对称氢硅化反应也具有较明显的催化作用,产物的化学产率为97%,ee值为35%。
4. 结论
(1) 以三乙四胺六乙酸为原料,在N2保护145℃下分别与(R)-或(S)-2-氨基-1-丁醇搅拌反应16 h,一步反应合成了目标物(R)-1或(S)-1。该方法在无溶剂条件下进行,后处理方便,产率较高。
(2) 配体(R)-1或(S)-1与[Rh(COD)Cl]2作手性催化剂,在苯乙酮与二苯基硅烷的不对称氢硅化反应中显示较明显的催化活性,但反应的对映选择性较低(最高仅为37%ee)。