1. 引言
Cu2O是一种重要的P型半导体,为赤铜矿结构,禁带宽度为2.0~2.2 eV [1] ,在超导[2] 、太阳能转换[3] 、锂电池负极材料[4] 、磁存储器[5] 、气敏传感器[6] 等方面有广泛应用。Cu2O还是一种稳定的光催化剂,它能在可见光辐照下使水分解成O2和H2。另外,Cu2O作为光催化剂还能够分解有机污染物,同时它还是高光吸收系数、低成本的光伏能量发生器的良好材料。基于Cu2O重要的应用价值、特殊性质,新颖、实用和可能的更大应用潜力[7] ,在材料制备研究中,控制合成均一形貌和尺寸的无机纳米粒子受到广泛关注。近年来,控制合成不同形貌的Cu2O已成为研究热点,并已成功合成Cu2O一维结构[8] -[10] 、薄膜[11] [12] 及多面体结构[13] -[15] 。Cu2O形状及维度对其应用有很大影响。Huang [9] 等制备了盒状、立方状、球状纳米Cu2O并且研究了它对不同染料的吸附特性。他们还制备了不同粒径的Cu2O并且比较了合成的Cu2O对甲基橙的吸附和光催化性能。不同维度的Cu2O比表面积和晶粒组成差异较大,对其性能有很大影响。不同多面体的组成晶面类型不同,而不同晶面的表面能、饱和程度、吸附能力及稳定性等各不相同,导致不同类型的多面体在性能上表现出较大的差异[16] -[18] ,增强了各形貌合成条件的可比性,有利于合成机理的深入研究。
标准摩尔生成焓是纳米材料固有的属性。纳米材料的形成过程是一个非常复杂的热力学和动力学过程,迄今为止,纳米材料热力学理论体系并没有正式建立,特别在热力学领域中,纳米材料的熵、焓、吉布斯自由能等热力学函数值的研究仍存在盲点。因此,如何设计实验、科学地获取纳米材料热力学函数值,进而探索纳米材料热力学函数与尺度、形貌、微观结构、生长取向的客观联系及演变规律,建立纳米材料基础热力学数据标准,成为“纳米材料热力学”研究亟待解决的课题。纳米氧化亚铜的标准摩尔生成焓、熵、Gibbs自由能等热力学函数值尚未见到报道。这就对其理论研究带来了不便,因此测量其标准摩尔生成焓等很有必要。
本文通过设计热力学循,对合成的四种同尺寸不同形貌纳米氧化亚铜的标准摩尔生成焓进行了测定,并总结了其规律。
2. 纳米氧化亚铜的合成
2.1. 试剂与仪器
试剂:氯化镁、氢氧化镁、氯化亚铜、酒石酸钾钠、硫酸铜、聚乙烯吡咯烷酮、乙酸铜,均为天津市福晨化学试剂厂产品,分析纯。聚乙二醇(生化纯、天津市福晨化学试剂厂)。乙醇、柠檬酸三钠、葡萄糖、无水乙醚、氢氧化钠均为北京化工厂产品,分析纯。
仪器:RD496-2000型微量热计(绵阳中物热分析仪器有限公司),XD-2 X射线衍射(北京普析通用),Quanta-400场发射扫描电镜(USA, FEI)。
2.2. 样品的制备
2.2.1. 球形纳米Cu2O的制备
将5 mmol乙酸铜和表面活性剂PVP 18 mmol,PEG 18 mmol溶于100 mL蒸馏水中,磁力搅拌15 min,确保其完全溶解,以一定速率向溶液中滴加35 mmol氢氧化钠溶液60 mL,搅拌5 min后加入5 mmol葡萄糖溶液40 mL,搅拌升温到70℃,反应5 min后移入70℃恒温水浴槽,继续反应25 min,取出,自然冷却至室温,将样品分别用蒸馏水、乙醇洗涤数次,60℃真空干燥3 h,可得纳米Cu2O球[19] 。
2.2.2. 立方块纳米Cu2O的制备
量取20 mL 0.7 mol/L硫酸铜和表面活性剂PEG 24 mmol加入到烧杯中,磁力搅拌下,将40 mL 0.6 mol/L氢氧化钠溶液加入到上述溶液中。磁力搅拌30 min,然后将70 mL 0.2 mol/L抗坏血酸逐滴加入到混合液中,磁力搅拌30 min,有黄色沉淀生成。离心过滤,用无水乙醇及去离子水洗涤数次,60℃下真空干燥8 h,得到纳米Cu2O立方块[20] 。
2.2.3. 六棱柱纳米Cu2O的制备
硫酸铜、葡萄糖、酒石酸钾钠按1:1。625:0.06摩尔比料液混合,加一定量OP稀释至100 mL,立即转入磨口锥形瓶,将磨口锥形瓶敞口放置水浴中,并在微波反应器内中心位置用高火加热至体系完全变红。取出后盖上瓶塞,放入95℃超级恒温槽陈化1 h,离心分离,产物依次用二次水、无水乙醇、无水乙醚洗涤,晾干,得到纳米Cu2O六棱柱[21] 。
2.2.4. 六角星纳米Cu2O的制备
将柠檬酸钠和硫酸铜以1:1的摩尔比溶解在去离子水中,配制成浓度为0.02 mol/L的Cu2+溶液30 mL。加入浓氨水使氨水和Cu2+摩尔比为2:1,用磁力搅拌器搅拌5 min。混合均匀后取40 mL移入反应釜中,加热至200℃,保温15 h。取出反应釜后自然冷却,将内衬中的样品离心分离,用去离子水冲洗2次,再用无水乙醇冲洗1次,最后于60℃干燥8 h,得到纳米Cu2O六角星[22] 。
以上Cu2O的合成参照了文献[19] -[22] ,相关条件进行了摸索和改良。
3. 纳米氧化亚铜的表征
四种不同形貌的纳米Cu2O的XRD图和SEM图分别如图1、图2所示,XRD图中所有的衍射峰都与Cu2O标准峰一致,图中较高的衍射强度和尖锐的衍射峰,表明所得产物的结晶性较好,未见有杂质峰,表明晶型单一、晶体有较高的纯度,根据半峰宽得到了晶体的粒径为20 nm。由SEM照片可知产物尺寸和形貌分布均匀,且尺寸大小与XRD结果对应。
4. 标准摩尔生成焓的测定和计算
对基准物质KCl在水中的溶解焓进行测量,以鉴定所使用的量热系统的准确度,表1是298.15 K下
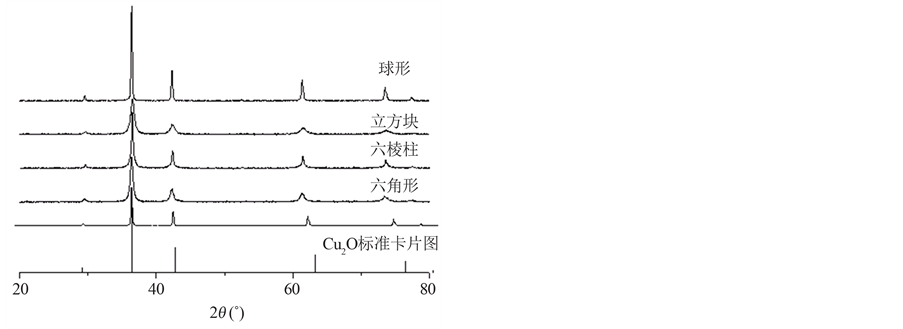
Figure 1. XRD spectra of four kind of nano cuprous oxide
图1. 四种纳米氧化亚铜的XRD图

 (a) (b)
(a) (b)
 (c) (d)
(c) (d)
Figure 2. SEM pictures of four kind of nano cuprous oxide (a, b, c, d were denoted to the ones of sphere, cube, hexagonal prism, hexagon)
图2. 四种纳米氧化亚铜SEM图(a、b、c、d分别为球形、立方块、六棱柱、六角形)
的测量数据。
由表1数据可知,量热系统测定KCl在去离子水中的溶解焓为(17.570 ± 0.02) kJ/mol,查阅文献[23] 知298.15 K下的溶解焓值为(17.518 ± 0.01) kJ/mol,二者的相对误差仅为0.297%,表明量热系统准确可靠。
因为焓是状态函数,故根据盖斯定律,设计了图3热循环过程[24] 。
由上述热循环可得出纳米氧化亚铜的标准摩尔生成焓,相关标准生成焓见文献[24] [25] ,计算公式如下:
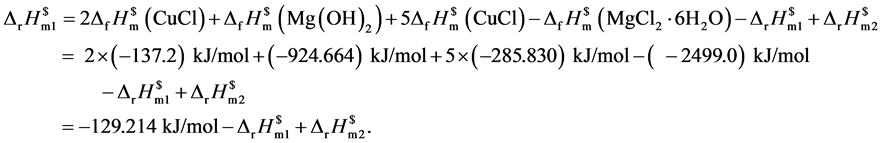
在298.15 K及常压下,将反应物和生成物均溶解于3.0 mL 1 mol/L的稀盐酸中,经过6次实验,得到粒径为20 nm的球状氧化亚铜的标准摩尔生成焓,其标准摩尔反应焓变见表2、表3。

Table 1. The solution enthalpy of KCl in water
表1. KCl在水中的溶解焓值数据

Table 2. The solution enthalpy of reactants with special nano cuprous oxide
表2. 粒径大小为20 nm球状纳米氧化亚铜反应物体系的溶解焓值数据
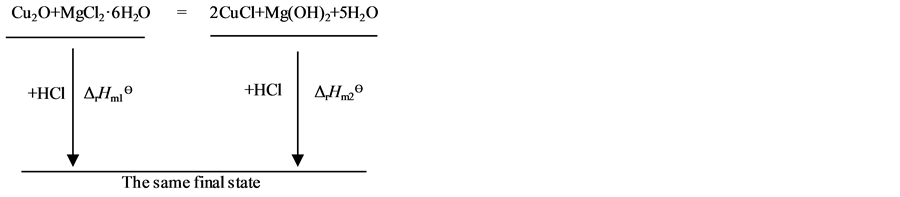
Figure 3. Thermochemical cycle of the reaction system of nano cuprous oxide
图3. 纳米氧化亚铜反应体系的热化学循环示意图
由热力学关系式计算得20 nm球状纳米氧化亚铜的标准摩尔生成焓为:
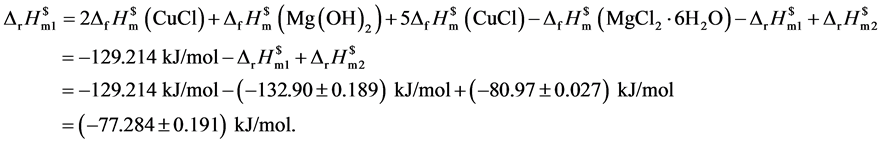
再设计热力学循环过程见图4。
由热力学状态函数的特性可得:

标准摩尔生成焓的计算
将40 mg其他形貌纳米氧化亚铜和40 mg球状纳米氧化亚铜分别与3 mL浓度1 mol/L的稀盐酸置于微量热仪中进行反应,两体系分别经过5次实验,其反应焓变、标准摩尔反应焓及平均焓值见表4。


Table 3. The solution enthalpy of products
表3. 生成物体系的溶解焓值数据
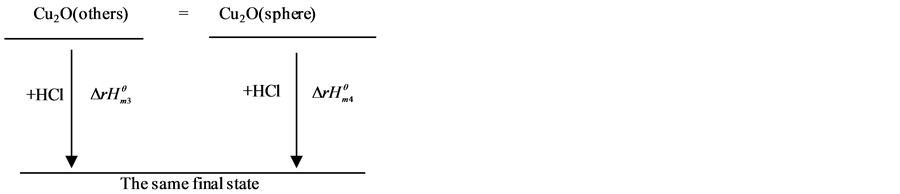
Figure 4. Thermochemical cycle of nano cuprous oxide of reaction system
图4. 氧化亚铜纳米反应体系的热化学循环示意图
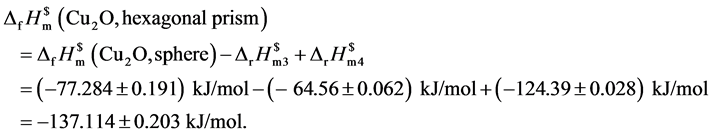
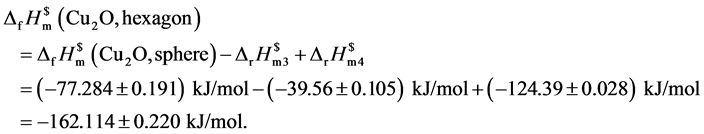
将以上数据汇总以形貌为纵轴,以对应的标准摩尔生成焓为横轴,绘制图5不同形貌与标准摩尔生成焓对应图。
5. 结果分析与讨论
测量数据表明处于纳米级氧化亚铜的标准摩尔生成焓相比较于文献[25] 给出的标准摩尔生成焓(−168.6 kJ/mol)变大。其中六棱柱、立方块的标准摩尔生成焓(−136.084 ± 0.194) kJ/mol、(−137.114 ± 0.203) kJ/mol相近这是因为其形貌同属柱类结构。六角形的标准摩尔生成焓最小(−162.114 ± 0.220) kJ/mol与文

Table 4. The standard molar enthalpy of formation of nano cuprous oxide with 20 nm and the ones of other
表4. 20 nm球状纳米氧化亚铜与其它形貌纳米氧化亚铜的溶解反应焓数据

Figure 5. Different morphology and standard molar enthalpy of formation
图5. 不同形貌与标准摩尔生成焓对应图
献给出的氧化亚铜的标准摩尔生成焓相近。根据Gibss热力学关系式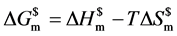 ,纳米氧化亚铜的稳定性是由
,纳米氧化亚铜的稳定性是由 决定的,熵必须加以考虑。纳米氧化亚铜形态不规则决定了其具有的熵值不同。熵是混乱度的标志,球和六角星熵值较大,因此焓值的大小并不能决定纳米氧化亚铜的稳定性顺序。
决定的,熵必须加以考虑。纳米氧化亚铜形态不规则决定了其具有的熵值不同。熵是混乱度的标志,球和六角星熵值较大,因此焓值的大小并不能决定纳米氧化亚铜的稳定性顺序。
基金项目
北京石油化工学院国家级大学生创新创业训练计划项目资助(2014J00007)。