1. 引言
四甲基二丙烯三胺是一种重要的化工原料,可作为聚氨酯催化剂,环氧树脂固化剂,环氧促进剂使用以及用于合成医药中间体和生产表面活性剂 [1] 。四甲基二丙烯三胺最初是二甲氨基丙腈加氢还原生成二甲氨基丙二胺的一种副产物 [2] ,国内对其研究较少。
关于四甲基二丙烯三胺文献较少,大多数都是专利报道。在德国专利DE-PS2624528中Leuckart- Wallach等人使用负载型催化剂催化合成四甲基二丙烯三胺,反应中需加入甲醛和甲酸,致使反应体系具有较强的酸性,该反应对设备有较强的腐蚀性,并且反应条件较为苛刻难以实现大规模生产 [3] 。根据美国专利U.S. Pat. No. 6391929报道,采用Raney Ni作为催化剂,该催化剂制作较为繁琐。在美国专利US
005101075A
中提到当载体是Al2O3时有利于二级胺的生成,尖晶石有利于三级胺的生成。其反应压力在5~30 MPa之间,反应压力过高对设备要求高且操作存在相当高的危险性,该方法二级胺和三级胺的选择性不高 [4] 。四甲基二丙烯三胺直接或间接的应用在生活、工业、交通以及医药等行业,其需求量也随着社会的发展而日益增加,生而产技术基本上都掌握在国外,因此关于四甲基二丙烯三胺的研究刻不容缓,同时此研究对C-N偶联反应也有一定的研究意义。
纳米金属催化剂具有较大的比表面积,表面原子配位不等使其具有较高的催化活性和选择性。由于纳米颗粒在热力学上不稳定,容易发生团聚而在工业中应用受到限制。因此,为了保证纳米粒子稳定性和金属粒子的分散度,我们将纳米金属负载在载体表面制作成负载型催化剂。γ-Al2O3是一种良好的催化剂载体,在工业中的广泛应用要归因于其良好的机械强度和热稳定性等优势。双金属催化剂能形成纳米金属合金,两种金属的协同作用,能有效的提高催化能力或产生新型催化活性。采用非贵金属代替部分贵金属,可减少贵金属的使用量,从而降低生产成本,因此双金属纳米金属催化剂受到广泛关注。在Marck课题组中的使用Pd-Ni双金属催化剂对氯硝基苯选择性加氢的实验中,催化剂显示出了良好的催化能力和选择性 [5] 。
本文以Pd-Ni/γ-Al2O3作为催化二甲基丙二胺与二甲基丙二胺加氢偶联反应的催化剂,催化剂采用浸渍法制作。实验过程中运用X射线衍射仪(XRD)、X射线光电能谱(XPS)以及透射电镜(TEM)对催化剂进行表征分析并确定催化剂的结构特点。与此同时,实验需对反应温度、氢气压力以及反应时间做研究探索并确定最佳反应条件。本文的研究目的是制作一种催化活性良好并且具有良好重用性的加氢偶联催化剂。
2. 实验部分
2.1. 主要仪器和试剂
主要实验仪器和试剂如表1和表2所示。

Table 1. List of reagents used in the experiment
表1. 实验过程中所用试剂列表

Table 2. List of main experimental instruments and equipment
表2. 实验过程中主要仪器与设备列表
2.2. 实验方法
2.2.1. 催化剂的制备
1) 催化剂前驱体的制备
Pd-Ni/γ-Al2O3催化剂由浸渍法制备 [6] - [9] 。以较为典型的催化剂Pd-Ni(1:2)/γ-Al2O3为例,制作过程如下:PdCl2 (88 mg)和Ni(NO3)2·6H2O (291 mg)溶解在10 mL去离子水中,在室温下不断搅拌(500 rmp)。待其充分溶解后,慢慢添加γ-Al2O3 (1.5 g)并持续搅拌(300 rmp) 5 h。完全浸渍后,在100℃下加热去除多余的水的粘性物质,并在真空干燥箱干燥(80℃) 12 h。
2) 催化剂的煅烧
将获得的催化剂前驱体研磨成粉,推入管式炉中,升温速率为5℃/min,然后保持在500℃维持4 h,整个过程中通入氮气(0.3 L/min)进行保护。达到设定温度通入氢气(0.15 L/min)对催化剂进行还原。最后获得Pd-Ni(1:2)/γ-Al2O3催化剂。为了研究金属负载量的影响,我们制备了不同的Pd/Ni摩尔比的催化剂用于加氢反应合成应用。
2.2.2. 实验内容
量取原料二甲氨基丙腈(25 mL)和二甲基丙二胺(29 mL),Pd-Ni(1:2)/γ-Al2O3催化剂 (0.9 g,原料总质量的2%),加入高温高压反应釜,组装反应釜,氢气排气三次,升压到指定气压(2.0 MPa),升温到指定温度(120℃),维持压强反应8 h,上述反应条件为最优条件,此外,其他因素影响和Pd-Ni(1:2)/γ-Al2O3催化剂的重复使用也做了一定的探究。
3. 结果与分析
3.1. 催化剂表征
3.1.1. X射线衍射
通过X射线衍射仪(XRD)对制备好的Ni/γ-Al2O3及不同Pd-Ni比例的Pd-Ni/γ-Al2O3催化剂进行了表征,表征结果如图1。图中显示了除了γ-Al2O3有较强的衍射峰外,样品中其他成分的衍射峰强度都普遍较弱 [10] 。在图1中分别在2θ = 32.8˚,37.4˚,45.8˚,67.2˚的位置上,可以明显观察到γ-Al2O3有四个衍射峰,分别对应着γ-Al2O3的(022),(025),(220)和(042)这四个位面(JCPDS 04-0877),在图1中(B)~(F) 2θ = 40.1˚的位置检测到了金属Pd的衍射峰,由图可知随着样品中的Pd含量的增加,Pd的衍射峰也明显增强。在所有样品中并没有检测到Ni组分的衍射峰,这可能是因为镍组分含量较少或者镍的分散性较好 [11] 。通过谢乐公式对催化剂中Pd的晶粒尺寸进行计算,所有结果均在8.0 nm左右。为了更好地确定Pd-Ni双金属催化剂的结构特征,需要进一步对催化剂进行其他的分析表征。
3.1.2. X射线光电子能谱
X射线光电子能谱仪(XPS)主要用于对样品中各种金属组分的电子特性进行分析。Pd/γ-Al2O3和不同Pd-Ni比例的Pd-Ni/γ-Al2O3催化剂的XPS图谱如图2所示,显示了不同催化剂的Pd 3d5/2结合能。图2中(A),在Pd/γ-Al2O3样品中检测到在335.1 ± 2 ev位置处有一结合能峰对应PdO。图2中(B)~(F)其他样品中结合能均在336.1 ± 1 ev位置处对应是PdO [5] [12] 。表明单金属催化剂Pd/γ-Al2O3中Pd以氧化态存在。根据文献报道,金属与载体之间强的相互作用会导致Pdn+的产生 [13] 。综上所述,在Pd/γ-Al2O3中金属Pd主要物相是PdO,所有Pd-Ni/γ-Al2O3催化剂中Pd主要以零价态存在。这种差异的存在表明金属Ni的加入有利于Pd的还原,且形成了Pd-Ni双金属纳米颗粒所致 [14] 。
Ni/γ-Al2O3和不同Pd-Ni比例的Pd-Ni/γ-Al2O3催化剂的XPS图谱如图3所示,图中显示不同催化剂
 (A) Ni/γ-Al2O3的XRD图谱;(B) Pd-Ni/γ-Al2O3 (1:3)的XRD图谱;(C) Pd-Ni/γ-Al2O3 (1:2)的XRD图谱;(D) Pd-Ni/γ-Al2O3 (1:1)的XRD图谱;(E) Pd-Ni/γ-Al2O3 (2:1)的XRD图谱;(C) Pd-Ni/γ-Al2O3 (3:1)的XRD图谱
(A) Ni/γ-Al2O3的XRD图谱;(B) Pd-Ni/γ-Al2O3 (1:3)的XRD图谱;(C) Pd-Ni/γ-Al2O3 (1:2)的XRD图谱;(D) Pd-Ni/γ-Al2O3 (1:1)的XRD图谱;(E) Pd-Ni/γ-Al2O3 (2:1)的XRD图谱;(C) Pd-Ni/γ-Al2O3 (3:1)的XRD图谱
Figure 1. X-ray diffraction of catalysts
图1. 催化剂的X射线衍射
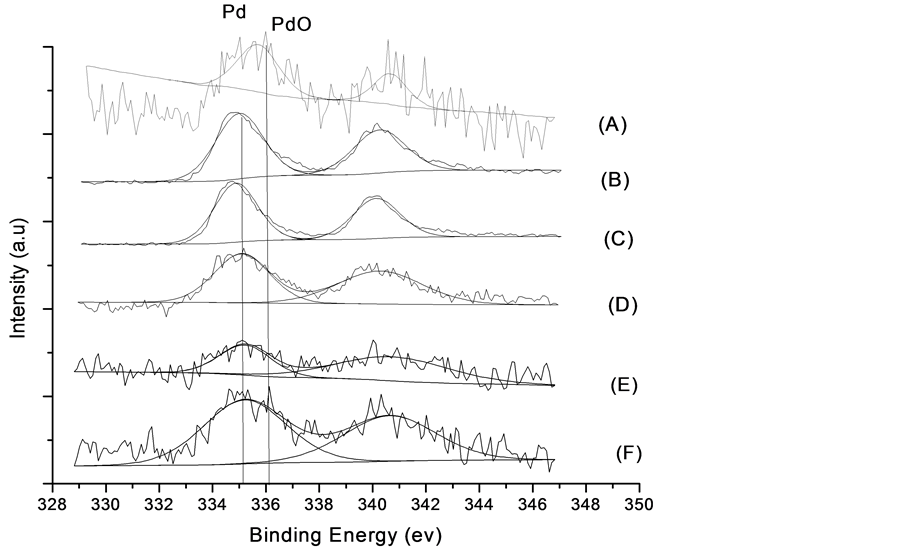 (A) Pd/γ-Al2O3的XPS图谱;(B) Pd-Ni/γ-Al2O3(3:1)的XPS图谱;(C) Pd-Ni/γ-Al2O3(2:1) 的XPS图谱;(D) Pd-Ni/γ-Al2O3(1:1) 的XPS图谱;(E) Pd-Ni/γ-Al2O3(1:2) 的XPS图谱;(C) Pd-Ni/γ-Al2O3(1:3) 的XPS图谱
(A) Pd/γ-Al2O3的XPS图谱;(B) Pd-Ni/γ-Al2O3(3:1)的XPS图谱;(C) Pd-Ni/γ-Al2O3(2:1) 的XPS图谱;(D) Pd-Ni/γ-Al2O3(1:1) 的XPS图谱;(E) Pd-Ni/γ-Al2O3(1:2) 的XPS图谱;(C) Pd-Ni/γ-Al2O3(1:3) 的XPS图谱
Figure 2. X-ray photoelectron spectroscopy of Pd
图2. Pd的 X射线光电子能谱
 (A) Ni/γ-Al2O3的XPS图谱;(B) Pd-Ni/γ-Al2O3 (1:3)的XPS图谱;(C) Pd-Ni/γ-Al2O3 (1:2)的XPS图谱;(D) Pd-Ni/γ-Al2O3 (1:1)的XPS图谱;(E) Pd-Ni/γ-Al2O3 (2:1)的XPS图谱;(C) Pd-Ni/γ-Al2O3 (3:1)的XPS图谱
(A) Ni/γ-Al2O3的XPS图谱;(B) Pd-Ni/γ-Al2O3 (1:3)的XPS图谱;(C) Pd-Ni/γ-Al2O3 (1:2)的XPS图谱;(D) Pd-Ni/γ-Al2O3 (1:1)的XPS图谱;(E) Pd-Ni/γ-Al2O3 (2:1)的XPS图谱;(C) Pd-Ni/γ-Al2O3 (3:1)的XPS图谱
Figure 3. X-ray photoelectron spectroscopy of Ni
图3. Ni的X射线光电子能谱
的Ni 2p3/2结合能主要分布在855.1 ± 0.2和856.2 ± 0.2 ev之间。前者对应的物相是NiO,后者为Ni2O3 [13] [15] [16] 。说明催化剂上的金属Ni部分被氧化。图中可以清晰观察到随着Pd量的增加Ni2O3结合能逐渐减弱,表明Pd抑制Ni2O3的形成。各个催化剂的Ni 2p3/2结合能存在着差异是由于Pd-Ni负载量的不同引起电子在Pd-Ni之间的转移数目有所不同而导致的 [17] 。
除了检测Pd 3d5/2和Ni 2p3/2结合能之外,XPS还对催化剂表面Pd和Ni的原子百分比进行了分析,结果见表3,从表3中可知,不同的催化剂中均含有金属Pd和Ni,证明了XRD中没有检测到的金属Ni确实是存在的,并没有在制作过程中全部丢失,可见在催化剂中Ni的分散性比较好以至于没有检测到Ni。从表中还可得知催化剂表面金属原子比例并不和金属负载量一致,可能是部分金属被引入了载体的内部或者被检测到的金属所覆盖。在所有催化剂中Pd-Ni/γ-Al2O3(1:2)催化效果最好,从表中结果推测,可能当催化剂表面原子接近1:1时,具有较理想的催化活性。
3.1.3. 催化剂的透射电子显微分析
为了进一步的确认催化剂Pd-Ni/γ-Al2O3的微观结构,我们对催化剂Pd-Ni/γ-Al2O3 (1:2)进行了透射电子显微镜(TEM)表征分析。如图4所示。图4(A)是催化剂颗粒整体电子图像,从图中可知,载体上颜色较深部分呈有规则椭圆形且较为均匀的分散在载体表面,可见类似椭圆黑色颗粒是活性组分并且确实被负载在载体之上。图4(B)是催化剂表面活性组分颗粒的电子图像,从图中可知活性组分颗粒直径约在10 nm左右。在图4(C)中,实验对催化剂表面活性颗粒直径进行了统计,结果显示活性组分粒径的范围在7~17 nm之间,平均尺寸在11.91 nm左右。图4(D)是催化剂表面原子所含原子种类总谱图及各种元素的对应的质量、原子百分比。再次证明了催化剂表面确实存在Pd和Ni。图4(E)和图4(F)是对催化剂表面原子进行了扫描,图4(E)是整体扫描图,图4(F)中分别是氧、铝、钯、镍各类元素的电子图像,对应的
表3. 催化剂XPS数据
a催化剂表面金属原子比例
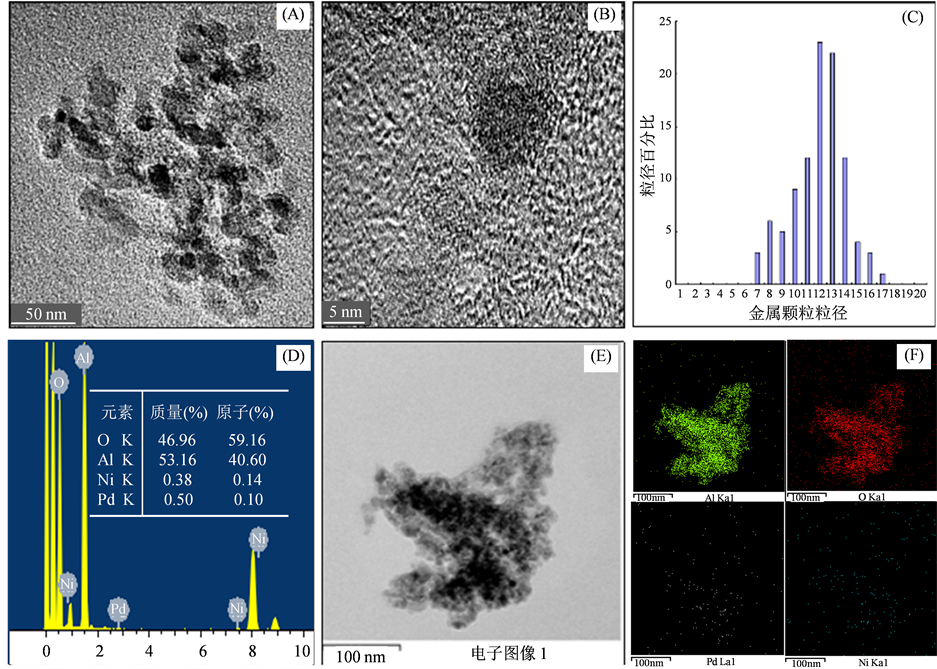 (A) 催化剂Pd-Ni/γ-Al2O3 (1:2)颗粒整体TEM图片;(B) 催化剂活性组分颗粒TEM图片;(C) 金属颗粒粒径分布图;(D) 催化剂Pd-Ni/γ-Al2O3 (1:2)的EDS图片;(E) 催化剂Pd-Ni/γ-Al2O3 (1:2) mapping图片;(F) 催化剂Pd-Ni/γ-Al2O3 (1:2)各种元素的mapping图片
(A) 催化剂Pd-Ni/γ-Al2O3 (1:2)颗粒整体TEM图片;(B) 催化剂活性组分颗粒TEM图片;(C) 金属颗粒粒径分布图;(D) 催化剂Pd-Ni/γ-Al2O3 (1:2)的EDS图片;(E) 催化剂Pd-Ni/γ-Al2O3 (1:2) mapping图片;(F) 催化剂Pd-Ni/γ-Al2O3 (1:2)各种元素的mapping图片
Figure 4. Transmission electron microscroph
图4. 透射电子显微镜图谱
颜色分别是红色、绿色、白色和蓝色。从图中可以清晰观察到载体不规则的簇拥在一起,Pd和Ni均匀的分散在载体表面。图5为四甲基二丙烯三胺合成反应式。
3.2. 反应催化剂的筛选
如表4所示,展示了Pd、Ni的单金属催化剂和不同Pd-Ni比例催化剂在不同反应条件下的反应结果。从表中可知,除了催化剂Ni/γ-Al2O3外其他催化剂均有催化活性。由此可见,Pd是催化剂必须的活性元

Figure 5. Reaction equation of the synthesis of 3,3'-iminobis(N,N-dimethylpropylamine)
图5. 四甲基二丙烯三胺合成反应式

Table 4. The catalyzing result of different types catalysts
表4. 不同种类催化剂催化结果
催化剂的用量为原料总量的2.0 wt%。不同钯镍比例的催化剂,比例中1为单位代表该组分是催化剂总量的0.5 wt%。Pd/γ-Al2O3催化剂中金属Pd负载量为0.5 wt% (相当于催化剂总质量);Ni/γ-Al2O3催化剂中金属Ni负载量为0.5 wt% (相当于催化剂总质量),混合催化剂Pd/γ-Al2O3和Ni/γ-Al2O3投入量为1.0 wt% (相对于二甲基丙二胺和二甲氨基丙腈总质量)。
素。从表中结果可以得出,催化剂Pd-Ni/γ-Al2O3 (1:2)最佳催化活性,根据表3可以推测催化剂表面金属原子比例非常重要,当催化剂表面Pd-Ni的原子比例接近1:1时催化活性最佳。相比较其他催化剂活性偏低可能是因为其表面金属原子比例偏离1:1而导致的。实验对催化剂Pd/γ-Al2O3的催化活性也做了验证,但是相比较Pd-Ni/γ-Al2O3 (1:2),不论是催化效率和选择性都大大的降低,可见金属Ni对于提升催化剂性能也是不可或缺的。实验中混合催化剂(Pd/γ-Al2O3 + Ni/γ-Al2O3)的催化活性并没有得到预想的结果,可见机械的混合催化剂并不能满足最佳催化剂的最佳活性结构。根据之前的表征结果,我们推测在催化剂表面Pd和Ni形成Pd-Ni双金属纳米颗粒的催化剂才具备最佳催化活性。
3.3. 压力对反应的影响
从表5中我们可以发现,当反应条件只有氢气压改变时,催化剂对四甲基二丙烯三胺的催化活性也随之改变,从0.5~2.0 MPa时,反应氢气压增加四甲基二丙烯三胺的产率也随着增加,当反应氢气压超过2.0 MPa时,四甲基二丙烯三胺的产率并没有增加反而有略微的降低。从表5中可以看出反应的压力对催化剂的催化活性有着直接的影响。反应中当氢气压力从0.5~2.0 MPa时,催化剂的选择性有着明显的提高,当压力超过2.0 MPa时,催化剂的选择性基本上没有什么变化。因此,我们推测反应中催化剂在2.0 MPa时,有着最佳的催化活性和选择性。
3.4. 反应温度对于反应的影响
从表6中我们可以发现,当反应条件只有温度发生改变时,催化剂对四甲基二丙烯三胺的催化活性也随之改变,从100℃~120℃时,随着反应温度增加四甲基二丙烯三胺的产率也随着增加,当反应温度

Table 5. Effect of different hydrogen pressures on reaction
表5. 不同氢气压力对反应的影响

Table 6. Effect of different temperatures on reaction
表6. 不同温度对反应的影响

Table 7. The result of recycling of catalyst
表7. 催化剂循环使用结果
超过
120 ℃
时,四甲基二丙烯三胺的产率并没有增加反而有略微的降低。反应中当温度力从110℃~120℃时,催化剂的选择性有着明显的提高,当压力超过
120 ℃
时,催化剂的选择性基本上没有什么提高反而下降。因此,我们推测反应中催化剂在
120 ℃
时,有着最佳的催化活性和选择性。
3.5. 催化剂的循环使用
在反应中,使用负载型双金属纳米催化剂催化二甲基丙二胺与二甲氨基丙腈合成四甲基二丙烯三胺。使用的钯镍双金属催化剂,其中钯是价格较为昂贵的贵金属,所以使用次数是衡量催化剂催化性能的关键之一。如果催化剂稳定性不高,催化剂在使用过程中就会失活而无法循环使用,就会大大的增加反应成本而加大推进工业化进程的难度。本论文在反应中对催化剂的稳定性也做了一定的研究。将最优催化剂Pd-Ni/γ-Al2O3 (1:2)投入到反应中,其他均为最优反应条件。反应结束后,将反应混合液进行过滤处理从而分离得到反应过后的催化剂,将回收得到的催化剂用丙酮从滤纸上冲洗下来,用丙酮将催化剂清洗5次,再用乙醇清洗3次,最后将催化剂置于
60 ℃
下的真空干燥箱内干燥12 h,将烘干后的催化剂回收,就可以重新投入到新的反应中,验证催化剂循环使用的稳定性。经过催化剂的循环使用实验发现,催化剂在多次循环使用之后仍能保持良好的催化活性和选择活性。催化剂循环使用结果在表7中,从表7中可以发现,催化剂在循环使用试验中反应条件均不变,四甲基二丙烯三胺的产率基本上没有发生改变,催化剂Pd-Ni/γ-Al2O3 (1:2)在连续使用5次之后仍能保持不变催化活性和选择性。在反应中回收过程有少量的催化剂损失,在投入反应时适当的减少反应的原料,使催化剂仍然为原料的2.0 wt%。综上所述,催化剂Pd-Ni/γ-Al2O3 (1:2)具有极强的稳定性,使催化剂可以多次循环使用。
4. 结果与讨论
通过大量的实验研究,我们开发了以γ-Al2O3为载体,负载Pd和Ni为活性组分的催化剂。实验证明该催化剂表面形成Pd-Ni双金属纳米合金,并且确定了最优反应条件。在最优条件下采用Pd-Ni/γ-Al2O3 (1:2)催化剂的四甲基二丙烯三胺的产率可达到40%,同时选择性大于90%。
致谢
在此感谢中国国家自然基金(Nos. 21571047, 21371044)的资金援助和高校新世纪优秀人才计划 (NCET-11-0627)的支持。