摘要:
SiO2抗结剂是一种白色蓬松粉末,能解决产品因吸潮受压形成的结块,同时具有吸附作用,吸湿或易从空气中吸收水分。主要被添加于颗粒、粉末状食品中防止颗粒或粉末食品的聚集结块,保持其松散或自由流动。本研究以水玻璃和硫酸为原料,在硫酸钠无机盐溶液中,在磁场作用下用酸化沉淀法制备二氧化硅抗结剂,控制反应的pH值为7~8,研究在不同温度下制得的SiO2抗结剂的收得率和粒度。结果表明,随着温度的升高,SiO2抗结剂的收得率逐渐增加,50℃时收得率仅为62.3%,温度达到90℃时,收得率达到87.2%;随着温度的升高,SiO2抗结剂的粒度增大,为了满足抗结剂粒度2~9 μm的要求,SiO2抗结剂的制备温度不宜超过70℃。
Abstract:
SiO2 anticaking agent is a kind of white fluffy powder, which can prevent the product from forming caking due to moisture absorption or pressure, and easy to absorb moisture from the air because of its adsorption. SiO2 anticaking agent is mainly added to particles and powder food to prevent particles or powder food aggregation, agglomeration, and maintain its loose or free flowing. In this study, sodium silicate and sulfuric acid were used as raw materials, SiO2 was prepared by acid precipitation under the action of magnetic field in sodium sulfate inorganic solution, the pH value of solution retained at 7 - 8, and the yield and grain size of the SiO2 anticaking agent at different temperatures were studied. The results showed that with the increase of temperature, the yield of SiO2 anticaking agent gradually increased, the yield is only 62.3% at 50˚C, and it reached to 87.2% at 90˚C; furthermore, with the increase of temperature, the particle size of SiO2 anticaking agent increased, and the particle size of the anticaking agent is 2 - 9 μm. In order to meet this requirement, the preparation temperature of the SiO2 should not exceed 70˚C.
1. 引言
抗结剂又称抗结块剂,是用于防止颗粒或粉末食品聚集结块,保持其松散或自由流动状态的食品添加剂。我国目前允许使用的抗结剂有亚铁氰化钾、硅铝酸钠、磷酸三钙、二氧化硅、微晶纤维素五种 [1] [2] [3] 。此外,国外允许使用的还有硅酸铝、硅酸铝钙、硅酸钙、硬脂酸钙、碳酸镁、硬脂酸镁、硅酸镁、高岭土、滑石粉和亚铁氰化钠等 [4] [5] [6] 。21世纪以来,随着食品行业的发展,抗结剂在食品领域的应用日益广泛,相关研究也越来越多。二氧化硅也称无定形二氧化硅,其性能特点是:严格的粒度分布、大的比表面积;高孔隙率、低堆密度;高透明度、高补强性;吸附力强等 [7] [8] 。制备二氧化硅抗结剂的方法可分为干式法和湿式法两种 [9] [10] 。干式法反应设备复杂,投资大,操作难度大。湿式法工艺简单,投资少 [11] [12] [13] 。本研究以水玻璃和硫酸为原料,在磁场作用下,在无机盐溶液中用湿式沉淀法 [14] [15] 制备二氧化硅抗结剂,控制反应的pH值保持在7~8,研究温度对二氧化硅抗结剂的收得率和粒度的影响。
2. 实验
2.1. 实验原料
本研究使用的实验原料及规格如表1所示。
表1. 试验中主要试剂
2.2. 实验流程
首先将水玻璃进行稀释,再用热水配置Na2SO4溶液,滴加两滴酚酞,在磁场作用下,在不同的温度下(50℃、60℃、70℃、80℃、90℃)恒温搅拌溶液,交替加入水玻璃和硫酸,使体系的pH值维持在7~8之间,水玻璃加完后,继续恒温搅拌10分钟,静置,抽滤出沉淀,然后用热水洗涤沉淀至无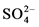 ,用BaCl2检验,将制得的沉淀在烘箱中烘干,研磨得成品。在磁场作用下,水玻璃和硫酸在硫酸钠无机盐溶液中发生的化学反应如下所示 [16] [17] [18] :
,用BaCl2检验,将制得的沉淀在烘箱中烘干,研磨得成品。在磁场作用下,水玻璃和硫酸在硫酸钠无机盐溶液中发生的化学反应如下所示 [16] [17] [18] :
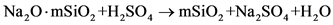 (1)
(1)
2.3. 研究方法
首先研究在磁场作用下,以水玻璃和硫酸为原料采用酸化沉淀法制取SiO2抗结剂的收得率;其次是采用马尔文激光粒度仪检测温度对制得的SiO2抗结剂粒度的影响。
3. 实验结果与讨论
3.1. 温度对SiO2抗结剂收得率的影响
由图1可知,在相同的磁场强度下,溶液温度在50℃~90℃区间,随着溶液温度的升高,SiO2抗结剂的收得率逐渐增加,50℃时收得率仅为62.3%,温度达到90℃时,收得率达到87.2%。
3.2. 温度对SiO2抗结剂粒度的影响
由图2可见,50℃、70℃、90℃下制得的粉体的粒度分布图均接近正态分布,由此说明该粉体的一致性好,粒度分布均匀。50℃下制得的粉体的平均粒径为7.82 μm,70℃下制得的粉体的平均粒径为8.96 μm,90℃下制得的粉体的平均粒径为11.75 μm,由此可见,溶液温度升高,沉淀析出的SiO2粒子碰撞聚集长大的活化能升高,从而使其粒径增大。本研究所制备的粉体用作食品抗结剂,要求其粒径为2~9 μm [19] [20] ,由此可知,在本实验条件下,70℃是制取SiO2食品抗结剂的最高温度。
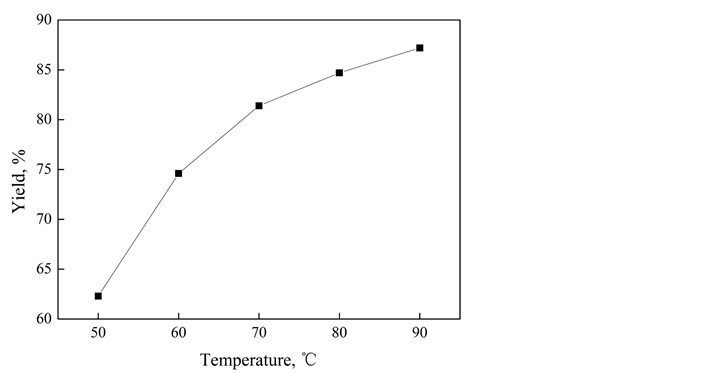
Figure 1. Yield of SiO2 at different temperatures
图1. 不同温度下SiO2的收得率
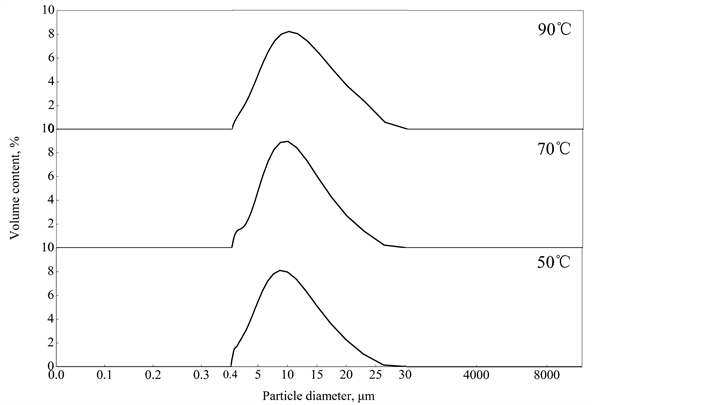
Figure 2. Grain size distribution of SiO2 anti caking agent at different temperatures
图2. 不同温度下SiO2抗结剂的粒度分布
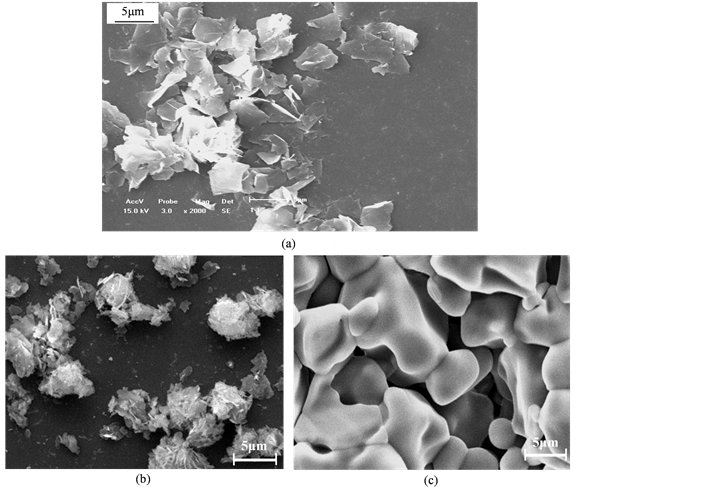
Figure 3. SEM photo of SiO2 anti caking agent prepared by acidification precipitation (a) Morphology of powders prepared at 50˚C; (b) Morphology of powders prepared at 70˚C; (c) Morphology of powders prepared at 70˚C after 800 × 30 mins corrosion
图3. 酸化沉淀法制取SiO2抗结剂的SEM照片(a) 50℃制得的粉体原貌、(b) 70℃制得的粉体原貌、(c) 70℃制得的粉体经800℃ × 30 mins热蚀后的形貌
3.3. 扫描电镜分析
由图3可见,在磁场作用下,在50℃下采用酸化沉淀法制得的SiO2抗结剂为片状堆积粉末,在70℃制得的是白色蓬松粉末,粒径在略大于5 μm。将70℃制得的粉末在800℃下热蚀30 mins,由其SEM照片可见,粉磨呈松散堆积的形态,将此粉末加入油粉产品、蛋白粉、骨粉、香水和其他食物中,可以解决此类产品由于吸湿结块的问题,从而保持其松散性或自由流动性。
4. 结论
本研究采用酸化沉淀法在磁场作用下制备SiO2抗结剂,研究溶液温度对制得的SiO2抗结剂收得率和粒度的影响。得出结论如下:
1) 在相同的磁场强度下,随着溶液温度的升高,SiO2抗结剂的收得率逐渐增加,温度升高到90℃时,收得率达到最大,为87.2%。
2) 温度升高,粉体的平均粒径增大,温度达到70℃时,粉体的粒径为8.96 μm,接近抗结剂要求粒径范围的上限,随后温度继续升高,所制得的粉体的粒径仍继续增大,不满足食品抗结剂要求的粒径为2~9 μm的条件,不能用作食品抗结剂。