1. 引言
越来越多的研究显示,蛋白质淀粉样纤维化是很多人类疾病的重要特征,例如,阿尔茨海默疾病、海绵性脑病和帕金森等均与相关的蛋白质和多肽的纤维化沉积有关[1] [2] 。目前已经发现30多种蛋白质与这些疾病相关,这些蛋白质并没有序列同源性,且表现出天然结构的多样性,但是形成的淀粉样纤维具有共同的β-折叠片结构[3] 。进一步的研究发现,一些与疾病不相关的蛋白质也可以形成淀粉样纤维,如乳球蛋白、溶菌酶等。目前的研究认为:淀粉样纤维化是所有蛋白质的共性,而与其特定的序列和结构无关[4] [5] 。
β-乳球蛋白是牛乳乳清蛋白中的主要成分,它是由乳腺上皮细胞合成的乳特有蛋白,是反动物和猪等乳中的主要乳清蛋白成分。β-乳球蛋白在牛乳中主要以二聚体形式存在,每个单体含有两个二硫键Cys66-Cys160和Cys106-Cys199还有一个Cys121自由硫氢基。β-乳球蛋白在中性pH时是由8条反平行链组成的桶状结构,桶外存在着一个具有3个转角的α螺旋和9条β链[6] [7] 。许多研究已经表明,β-乳球蛋白在酸性条件下加热或者在中性条件下加入变性剂时很容易形成淀粉样纤维[8] -[10] 。
二硫苏糖醇(Dithiothreitol,简称为DTT)是一种小分子有机还原剂,DTT可以作为巯基化DNA的还原剂和去保护剂。巯基化DNA末端硫原子在溶液中趋向于形成二聚体,特别是在存在氧气的情况下。这种二聚化大大降低了一些偶联反应实验(如DNA在生物感应器中的固定)的效率;而在DNA溶液中加入DTT,反应一段时间后除去,就可以降低DNA的二聚化。DTT也常常被用于蛋白质中二硫键的还原,可用于阻止蛋白质中的半胱氨酸之间所形成的蛋白质分子内或分子间二硫键。但DTT往往无法还原包埋于蛋白质结构内部(溶剂不可及)的二硫键,这类二硫键的还原常常需要先将蛋白质变性(高温加热或加入变性剂,如6M盐酸胍、8M尿素或1% SDS)。反之,根据DTT存在情况下,二硫键还原速度的不同,可以判断其包埋程度的深浅。
本文研究了二硫苏糖醇对乳球蛋白淀粉样纤维形成的影响。实验结果表明,二硫苏糖醇能够抑制乳球蛋白淀粉样纤维的形成,且这种抑制效应依赖于二硫苏糖醇的浓度,当二硫糖醇浓度达到10 mM时,乳球蛋白淀粉样纤维的形成能够完全被抑制。
2. 实验方法和步骤
2.1. 试剂与仪器
1) 试剂:β-乳球蛋白、硫黄素-T(Thioflavin)、刚果红、二硫苏糖醇均为Sigma公司产品,其它试剂均为国产分析纯试剂。实验过程中所用水均为MilliQ去离子水,所有缓冲溶液均用去离子水配制。
2) 仪器:F-4600荧光光谱(日本Hitachi公司),Shamizu UV-1800型紫外可见分光光度计,H-7650型透射电子显微镜。
2.2. 淀粉样纤维样品的制备
配制浓度为10 mM的磷酸钠缓冲液(pH 7.0),称取β-乳球蛋白配制蛋白质母液,后用0.22 μm的无菌注射过滤器过滤以除去颗粒状物质。再称取尿素配制10M的变性剂母液。把β-乳球蛋白和尿素溶液进行混合得到5M的蛋白和变性剂混合溶液,然后再称取一定量的二硫苏糖醇配制溶液,把该二硫苏糖醇母液加入到蛋白和变性剂的混合溶液,最终得到含有不同浓度二硫苏糖醇的混合溶液,用膜封好管口,置于37℃的水浴中进行培养,在不同的时间点取出一定量的β-乳球蛋白样品进行检测,剩下样品溶液封口后继续恒温培养。
2.3. ThT荧光检测
使用10 mM,pH值7.0磷酸钠缓冲液配制1 mM ThT的储液。取不同时间的β-乳球蛋白样品10 μL,加入ThT储液使其最终的浓度为20 μM,与10 mM,pH值为7.0磷酸钠缓冲液混合使其体积为3 mL,置于水浴中37℃保温。用F-4600型荧光光谱仪检测482 nm的荧光强度值,选择荧光激发波长为450 nm,激发光栅长度为5 nm,发射光栅长度也为5 nm。连续检测60 s荧光强度取平均值来增加检测的信躁比,同时扣除空白对照的平均值。
2.4. 刚果红结合
用10 mM,pH值为7.0磷酸钠缓冲液配制浓度为200 μM的刚果红储液,加入10%(v/v)的无水乙醇作为助溶剂。后用022 μm的无菌注射过滤器过滤以除去颗粒状物质。储液4℃避光保存。取在不同二硫苏糖醇浓度条件下培养的β-乳球蛋白样品,加入刚果红储液,然后用Shamizu UV-1800型紫外可见分光光度计扫描400 nm~700 nm,对照组为只含有刚果红和只含有β-乳球蛋白的磷酸纳缓冲液。
2.5. 透射电子显微镜
在不同的时间将待测的β-乳球蛋白淀粉样纤维的样品轻微振荡均匀,取样品5 μL加到聚醋酸甲基乙烯酯膜覆盖的铜网(200孔)上,用2%的磷钨酸负染40 s,多余染液和样品用滤纸吸干。在H-7456型透射电子显微镜下观察,加速电压为80 V。
3. 结果
3.1. 二硫苏糖醇对β-乳球蛋白淀粉样纤维形成的影响
3.1.1. 动力学影响的研究
由于ThT是一种能与淀粉样纤维结合从而导致其荧光强度升高的荧光染料,因而ThT已经被广泛地用于淀粉样纤维形成动力学的研究[11] -[13] 。二硫苏糖醇对β-乳球蛋白淀粉样纤维形成的影响如图1所示。由图1可见,在蛋白溶液中不存在二硫苏糖醇的情况下,β-乳球蛋白在5M的尿素溶液中(37℃)培养到12天左右时,当加入ThT时,其荧光强度可是明显的增强,这表明β-乳球蛋白在部分解折叠的情况下能够形成淀粉样纤维。同时我们也可以观察到荧光增加的曲线是S型的,这说明其淀粉样纤维化是一个成核-聚合的动力学过程。然而,在蛋白溶液中存在5M的二硫苏糖醇时,虽然ThT荧光强度仍然能够上升,但淀粉样纤维形成的滞后时间增加,荧光上升的强度明显的减小,这表明二硫苏糖醇能够抑制β-乳球蛋白淀粉样纤维的形成。特别当二硫苏糖醇的浓度增加到10 mM时,即使把蛋白溶液培养到60天
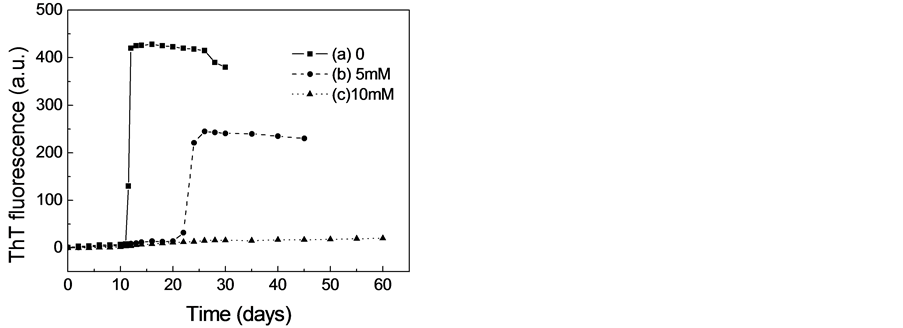
Figure 1. Effect of DTT on amyloid fibril formation of β-lactoglobulin monitored by ThT fluorescence. (a) 0 (b) 5 mM (c) 10 mM DTT
图1. ThT荧光检测二硫苏糖醇对β-乳球蛋白淀粉样纤维形成的影响 (a) 0 (b) 5 mM (c) 10 mM DTT
时,ThT荧光的强度也不会增强,这表明10 mM的二硫苏糖醇能够完全抑制β-乳球蛋白淀粉样纤维的形成。
3.1.2. 刚果红结合实验
由于刚果红可以和淀粉样纤维发生相互作用,导致刚果红吸收光谱的红移,因此常被用来检测淀粉样纤维的存在[14] 。为了探索二硫苏糖醇是否能对β-乳球蛋白淀粉样纤维的形成产生抑制效应,我们又使用刚果红结合实验分析了当加入二硫苏糖醇时β-乳球蛋白淀粉样纤维的形成情况。图2展示出刚果红吸收谱的变化。当溶液中不存在纤维时,刚果红在490 nm出有一个吸收峰,而当刚果红与在5M尿素中培养12天的β-乳球蛋白溶液培养时,其吸收峰红移到了504 nm处,并且在540 nm处出现了一个肩峰,这种吸收谱的变化表明在这种条件下β-乳球蛋白形成了淀粉样纤维。然而,当β-乳球蛋白在10 mM的二硫苏糖醇中培养时,刚果红的吸收峰没有出现明显的红移,与ThT荧光的结果一样,这个结果进一步支持了我们得出的结论:10 mM的二硫苏糖醇能够完全抑制β-乳球蛋白淀粉样纤维的形成。
3.1.3. 透射电子电镜
为了表明二硫苏糖醇对β-乳球蛋白淀粉样纤维组装的抑制效应,我们使用透射电镜直接观察了β-乳球蛋白当溶液中存在不同浓度的二硫苏糖醇时淀粉样纤维的形成情况。图3展示出当不存在二硫苏糖醇时,β-乳球蛋白所形成淀粉样纤维的形貌,成熟的β-乳球蛋白的淀粉样纤维展示出典型的淀粉样纤维形貌,其特征是又长又直的密集的直径为8~30 nm的纤维。当存在5 mM二硫苏糖醇时,β-乳球蛋白展示出与控制样品相同的形貌,但与控制样品比较起来,其纤维的数量明显的减少了,这表明二硫苏糖醇能够抑制β-乳球蛋白淀粉样纤维的形成,特别是当二硫苏糖醇的浓度达到10 mM时,在电镜下没有观察到淀粉样纤维,这表明,10 mM的二硫苏糖醇完全抑制了β-乳球蛋白淀粉样纤维的形成。
4. 讨论
β-乳球蛋白是具有淀粉样纤维化倾向的蛋白之一,酸化、升高温度和加入变性剂等因素都能够导致β-乳球蛋白形成淀粉样纤维,形成的纤维形貌依条件不同而具有多样化特征。在本实验中,β-乳球蛋白在低和高浓度的尿素中均不能够形成淀粉样纤维,而在中等浓度的尿素中形成了淀粉样纤维,这个结果表明:部分解折叠态的β-乳球蛋白是淀粉样纤维的前体。
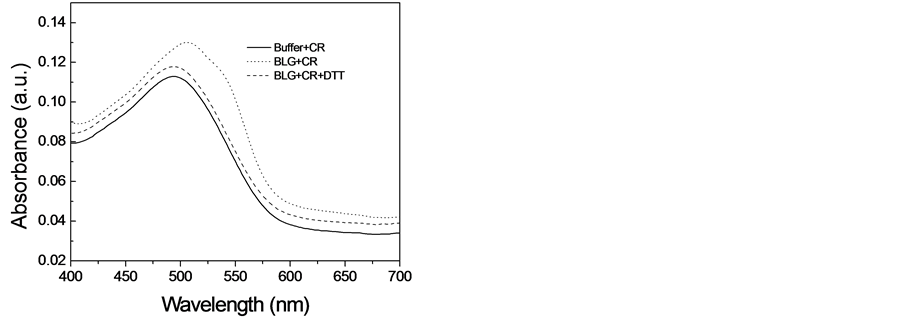
Figure 2. Congo Red binding assays to characterize the effect of DTT on amyloid fibril formation of β- lactoglobulin
图2. 刚果红连接确定二硫苏糖醇对β-乳球蛋白淀粉样纤维形成的影响
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Transmission electron microscopy of amyloid fibrils of β-lactoglobulin. (a) 0 (b) 5 mM (c) 10 mM (Scale bars represent 200 nm)
图3. 不同DTT浓度下β-乳球蛋白淀粉样纤维的透射电镜形态图 (a) 0 (b) 5 mM (c)10 mM (标尺代表200 nm)
二硫键在蛋白质折叠和稳定性方面扮演了非常重要的作用。但二硫键在蛋白质淀粉样纤维形成过程中的作用却是有争议的[15] [16] 。Yamamoto [17] 等人已经展示出还原剂,例如二硫苏糖醇和半胱氨酸,能够抑制微球蛋白淀粉样纤维的形成,这表明二硫键在微球蛋白淀粉样纤维形成过程中起着至关重要的作用,然而来鲁华[18] 等人的研究展示出,溶菌酶在被完全还原时也能够形成淀粉样纤维。这表明二硫键在溶菌酶淀粉样纤维形成过程中没有作用。然而在我们的实验中,当在部分解折叠态的β-乳球蛋白溶液中加入二硫苏糖醇时,其淀粉样纤维的形成受到了抑制,并且这种抑制效应是与二硫苏糖醇的浓度相关的。当其浓度达到10 mM时,淀粉样纤维的形成被完全抑制。以前的研究表明,二硫苏糖醇能够还原部分解折叠β-乳球蛋白中二硫键。因此,我们的结果就证明了:当β-乳球蛋白中的二硫键被破坏时,其淀粉样纤维的形成就会受到抑制,这表明,二硫键在β-乳球蛋白中淀粉样纤维的形成过程中扮演了关键的作用。
综上所述,β-乳球蛋白在中等浓度的尿素溶液中时能够形成淀粉样纤维。二硫苏糖醇对该过程有明显的抑制作用。本研究的结果对于了解β-乳球蛋白淀粉样纤维形成的机制,以及寻找抑制蛋白淀粉样纤维形成的小分子化合物提供了实验依据。
致 谢
感谢中央高校基本科研业务费(KYZ201321)的支持。