1. 引言
NiFe2O4是微波、电工等领域常用的软磁铁氧体材料[1] [2] 。由于纳米材料具有独特的物理化学性能,与块体材料相比,其光、电、磁、热等性能有较大的不同,因此近年来对纳米尺度NiFe2O4尖晶石粉体的制备技术及性能有了一定的研究。目前制备NiFe2O4尖晶石纳米粒子的主要方法有溶胶-凝胶法[3] 、水热法[4] 、共沉淀法[5] 等。低温固相反应法是近年来迅速发展起来的纳米材料合成领域的一个小小分支,该方法具有不使用溶剂、高选择性、高产率、污染少、节省能源、合成工艺简单等优点[6] ,符合当今社会绿色化学发展的要求,因而成为纳米材料合成方法的一个重要选择。
煅烧温度对粉体的相组成、粒径分布和形貌具有重大影响。改变煅烧温度能够影响热处理过程中反应驱动力,从而影响成核速率和晶体生长速率,进而影响到粉体的晶粒大小与形貌。本文采用低温固相合成法制备NiFe2O4纳米粉,以NaCl为分散剂,先通过研磨反应制备前驱体,再将前驱体在一定温度下煅烧保温即得到NiFe2O4纳米粉。重点考察煅烧温度对分析物相与形貌的影响,为制备工艺优化提供数据参考和理论依据。
2. 实验方法与过程
采用低温固相反应法合成NiFe2O4纳米粉。首先将反应物FeSO4∙7H2O、NiSO4∙6H2O和NaOH以及分散剂NaCl分别充分研细,以增加反应物的相互接触面积,增强反应活性;按FeSO4∙7H2O:NiSO4∙6H2O:NaOH摩尔比为2:1:6称取原料50 g和占总质量20%质量分数的分散剂NaCl置于玛瑙研钵中,充分混合,均匀用力研磨,随着研磨的进行,开始阶段反应剧烈,反应物中的结晶水析出,由开始的浅绿色粉末状变为棕黑色黏液状,并且有少量的水蒸气产生,约10 min后反应趋于缓和,慢慢地由黏液状变为黏块状,继续研磨后变干,最后得到棕褐色块状前驱体。然后将前驱体分别在600℃、700℃、800℃和900℃煅烧保温1小时后洗涤干燥得到NiFe2O4粉体。采用荷兰PANalytical公司生产的X’ Pert Pro型粉末X-射线衍射仪(XRD)对样品进行物相分析,采用Cu靶,步长为0.02,电流为40 mA,电压为40 KV。采用日本SHIMADZU公司生产的SSX-550型扫描电子显微镜(SEM,加速电压为15 kV)和美国FEI公司生产的Tecnai G220型透射电子显微镜(TEM,加速电压为200 kV)表征所得粉体的形貌。
3. 结果与讨论
3.1. 煅烧温度对粉体物相的影响
图1为不同煅烧温度下得到样品的XRD图谱。从图中可以得知,不同温度下煅烧得到的样品的衍射峰均与NiFe2O4标准卡片XRD图谱相(JCPDS No. 01-086-2267)相一致,说明不同温度下煅烧得到的粉末均属于立方晶系,属于Fd3m空间群。同时,随着煅烧温度的提升,衍射峰强度逐渐增强,衍射峰逐渐变得尖锐,晶化特征逐渐加强,说明粉体的结晶度变好,粉体的晶粒尺寸增大。
对于纳米材料,可以用谢乐(Scherrer)公式[7] 来计算平均晶粒尺寸。谢乐公式表达式如下所示:
 (1)
(1)
其中d为平均晶粒尺寸,nm;B为Scherrer常数,在本实验中等于0.89;λ为X-射线(Cu Kα)波长,为0.154056 nm;β为积分半高宽度(弧度制rad);θ为衍射角。
根据XRD图谱中样品最强衍射峰(311),采用谢乐公式(式1)计算不同煅烧温度下粉体的晶粒尺寸,计算结果如表1所示。结果表明,煅烧温度从600℃增至800℃时,晶粒尺寸仅从28.0 nm增加到41.0 nm,但煅烧温度达到900℃时,晶粒尺寸急剧增大,达到80.1 nm,几乎增长一倍。
由晶体生长动力学理论可知[8] [9] ,物质晶化过程中晶粒生长速率为:
 (2)
(2)
其中: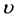 为晶粒生长速率;A为常数;E为晶粒长大激活能;R为理想气体常数;T为热力学温度;
为晶粒生长速率;A为常数;E为晶粒长大激活能;R为理想气体常数;T为热力学温度;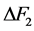 为非晶态与晶态之间摩尔自由能差。一般情况下,相变驱动力很大,即
为非晶态与晶态之间摩尔自由能差。一般情况下,相变驱动力很大,即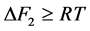 。假设晶粒以恒速生长,则式(2)可以简化为
。假设晶粒以恒速生长,则式(2)可以简化为
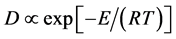 (3)
(3)
当热处理时间相同时,式(3)可以写为
 (4)
(4)
两边同时取自然对数,得到如下表达式:
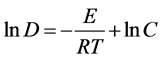 (5)
(5)
其中D为晶粒尺寸,C为常数。从式(4)可知,晶粒尺寸D与1/T呈指数关系。从式(5)可以得知,lnD与1/T成线性关系,斜率为 。计算出各温度下的lnD数值,绘制出lnD~1/T关系曲线,可得到直线的
。计算出各温度下的lnD数值,绘制出lnD~1/T关系曲线,可得到直线的
斜率值S;然后根据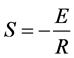 可计算出热处理过程中晶粒长大的活化能E (kJ∙mol−1)。考虑到煅烧温度为
可计算出热处理过程中晶粒长大的活化能E (kJ∙mol−1)。考虑到煅烧温度为
900℃时晶粒异常长大,因此煅烧温度只考虑600℃、700℃和800℃这三个温度。图2为NiFe2O4晶粒生长拟合曲线,其中斜率S为−1774.94。根据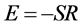 计算得到NiFe2O4晶粒在煅烧热处理过程中的晶粒长大活化能为14.76 kJ∙mol−1。这表明热处理过程中晶粒长大主要以界面扩散为主[10] 。
计算得到NiFe2O4晶粒在煅烧热处理过程中的晶粒长大活化能为14.76 kJ∙mol−1。这表明热处理过程中晶粒长大主要以界面扩散为主[10] 。
3.2. 煅烧温度对粉体形貌的影响
图3和图4分别为不同煅烧温度下所得样品的TEM和SEM照片。煅烧温度为600℃时,相对独立的颗粒大小相对比较均衡,颗粒主要呈圆球形,粒径分布相对较窄,颗粒粒径主要分布在10~45 nm之间;有部分非晶形的物质连成一片,在TEM照片中特别明显,形成一片黑团块,其他非晶形的物质分散在独立的颗粒之间,加大颗粒间的团聚。煅烧温度为700℃时,颗粒分布相对较广,小颗粒主要呈球形,有些小颗粒相互聚集成颗粒团,大颗粒主要是多面体形状。当煅烧温度提升到800℃,颗粒主要呈多面体
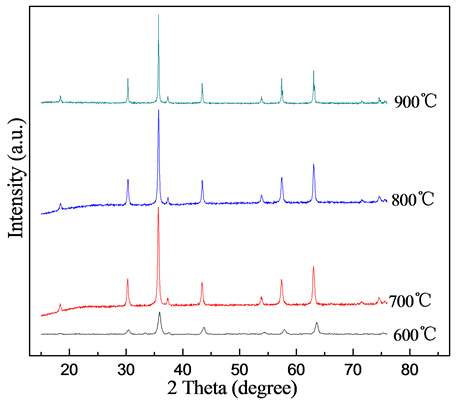
Figure 1. XRD patterns of samples prepared by various calcination temperatures
图1. 不同煅烧温度下试样的XRD图谱
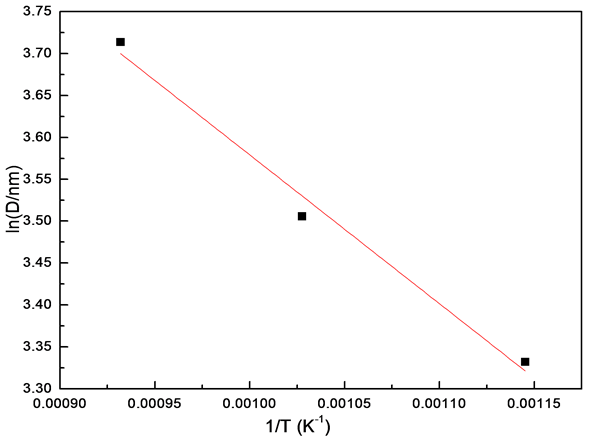
Figure 2. Fitting curve for grain growth of NiFe2O4 nanocrystalline
图2. NiFe2O4晶粒生长拟合曲线

Table 1. The crystallite sizes of NiFe2O4 powders under different calcination temperatures
表1. 不同煅烧温度下粉体的晶粒尺寸
形状,颗粒粒径分布相对较窄,主要在30~60 nm之间,颗粒分散性相对较好。煅烧温度达到900℃时,颗粒明显长大,部分颗粒粒径超过100 nm,颗粒多边化明显。
多组分固相化学反应开始于两相的直接接触部分,反应产物层一旦生成,为了使反应继续进行,反应物需要以扩散方式通过生成物层进行物质传输,而这种扩散对大多数固体是较慢的,而温度对物质的扩散起着至关重要的作用。温度越高,物质扩散速率越快。反应物只有集积到一定大小时才能成核,并

Figure 3. Fitting curve for grain growth of NiFe2O4 nanocrystalline
图3. NiFe2O4晶粒生长拟合曲线
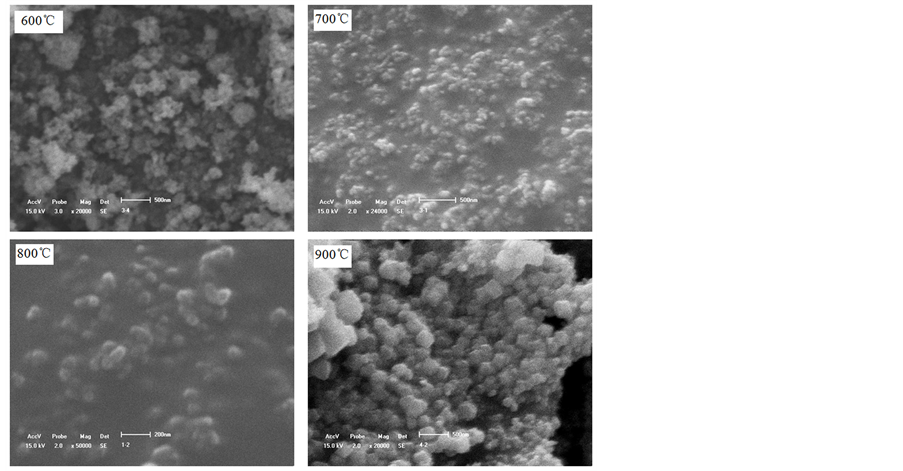
Figure 4. SEM photos of samples prepared by various calcination temperatures
图4. 不同煅烧温度下试样的SEM照片
且成核需要一个温度,低于某一温度Tn,反应则不能发生,只有高于Tn时反应才能进行。这种固体反应物间的扩散及产物成核过程便构成了固相反应特有的潜伏期[6] 。这两种过程均受温度的显著影响,温度越高,扩散越快,产物成核越快,反应的潜伏期就越短;反之,则潜伏期就越长。当低于成核温度Tn时,固相反应就不能发生。
煅烧温度为600℃时,反应温度低,反应驱动力小,晶粒生长缓慢,颗粒主要呈球形,并存在大量微晶,团聚严重。在煅烧温度为700℃时,温度相对较低,反应驱动力相对较小,物质扩散相对较慢,潜伏期较长,晶粒生长速率不一致,先生成的晶粒以迁移方式相互聚集,通过晶界迁移方式长大成大颗粒并且多边化,以多边体形貌存在,后生成的晶粒来不及生长长大以初生的圆球形颗粒存在。因此,煅烧温度为700℃,从TEM照片中可以看到小颗粒呈圆球形、大颗粒呈多边体形。当煅烧温度为800℃时,反应驱动力相对较大,反应物的扩散接触速度加快,反应速度相应较快,潜伏期缩短,在适当的保温时间内反应充分进行,NiFe2O4晶粒陆续生成,前后相差的时间不大,因此晶粒几乎都能在给定的保温时间内生长成单个颗粒,先生成的颗粒稍能长得大些,因而颗粒的表面活性相对较低,团聚相对较少,颗粒主要呈多边体形。当煅烧温度为900℃,温度高反应驱动力大,潜伏期进一步缩短,物质扩散明显加快,反应速率快,在保温时间内NiFe2O4微晶能全部生长成单个颗粒,并且先生成的NiFe2O4晶粒能够在余下的保温时间内以迁移方式相互聚集,通过晶界迁移方式长大成大颗粒并且多边化,有大颗粒生成,如900℃时煅烧得到样品的电子照片所示。
4. 结论
1) 煅烧温度升高,衍射峰强度增强,晶化特征明显,粉体平均晶粒尺寸增大;
2) NiFe2O4在煅烧热处理过程中的晶粒长大活化能为14.76 kJ∙mol−1,晶粒长大主要以界面扩散为主;
3) 随着煅烧温度的提高,颗粒从圆球形向多边体形转变,晶粒明显长大,分散性变好。
基金项目
中国博士后科学基金资助项目(2014M551109);中央高校基本科研业务费专项资金资助项目(N140203004)。