1. 引言
燃料电池是一种对环境污染低、燃料利用率高、经济效益好的能量转换设备,成为缓解能源短缺的一个重要的替代方案。质子交换膜燃料电池(PEMFC)的核心部件是质子交换膜。因铂的稳定性高,耐腐蚀性强,较好的电催化活性,及与吸附质成键能力强等优越性能,是优良的燃料电池阳电极材料,常常被用于质子交换膜中。在质子交换膜燃料电池中,最理想的燃料是氢气;而目前工业氢气通常是通过碳氢化合物裂解而制得,其中含有混合气体CO。在酸性电解质中,CO会优先吸附在Pt表面的活性位且难以脱附,从而大大降低了氢气的吸附量[1] ,导致催化剂Pt发生“CO中毒”效应。因此研究新型抗CO中毒的催化剂显得十分重要。
由于铂金的优良性能,开发以铂金材料为基础的新型催化材料,使其既能防止催化剂中毒,又具备有效催化氢或其它碳氢化合物氧化的阳极材料,成为当前研制催化材料的重要方向。研究学者们试图通过金属掺杂来改善催化剂的抗CO中毒性能,如在铂中加入一种或多种金属(Mn、Ru、Sn,Pd等) [2] - [4] ,制备铂合金以期得到性能优良、使用寿命长的催化剂材料。研究表明,合金的表面形貌、聚合方式、催化剂载体对合金催化剂的催化活性与稳定性具有重要影响。通过研究多种铂合金对含有CO的氢气的催化反应,Wantanabe等人指出PtMo,PtCo,PtNi以及PtFe等合金都具备一定的抗CO中毒能力 [5] - [7] 。在理论上,Gong等人系统地研究了CO在过渡金属氧化物如PdO2,RuO2,RhO2,OsO2和PtO2表面上的氧化,发现CO在过渡金属氧化物表面上的氧化活化能比相应的金属表面上的氧化活化能低得多 [8] 。Christoffersen [9] 等利用DFT计算,从CO和H竞争吸附的角度出发,研究了混合气体在PEMFC阳极表面的催化反应。他们指出在CO和氢气共存的环境中,PEMFC阳极表面对CO和H的吸附存在选择性,且一定条件下可使H吸附量最大化。这些研究表明在开发抗CO中毒和催化氢气氧化性能高的合金催化剂过程中,了解吸附质与催化材料相互作用原理是改善质子交换膜性能的重点,对研制可调控CO吸附能力的催化材料具有非常重要的实用价值。
Stadler [10] 发现铁电材料的极化效应不仅可以改变负载金属薄膜的性质,而且不同的极化方向可对化学反应速率产生显著的影响。诸多研究表明利用铁电材料极化效应是实现调控催化反应速率的有效手段。BaTiO3表面负载Ni催化乙烯加氢的研究 [11] [12] 表明,BaTiO3极化和结构相变对表面Ni催化活性产生重要影响。由于极化电场的存在,LiNbO3负载Cu和Au对乙醇氧化反应的活性明显增强 [13] 。为探讨铁电薄膜极化效应对铂金薄膜催化性能的影响,在本文中,我们采用第一性原理着重讨论了BaTiO3和PbTiO3的铁电极化对CO吸附的调控作用。
2. 计算方法与模型
本文计算使用基于密度泛函的VASP软件包计算完成。计算中采用投影缀加平面波方法(PAW)描述离子实与价电子之间的相互作用,而交换关联函数采用GGA-PBE,平面波截断能为450 eV。计算中采用6 × 6 × 1的Monkhorst-PackK点网格 [14] ;结构优化的收敛标准为总能收敛值1.0 × 10−4 eV和任意原子所受的Hellmann-Feynman力均小于0.01 eV/Å。
计算表明铁电相BaTiO3和PbTiO3的晶格常数分别为a = 3.99 Å,c = 4.33 Å和a = 3.90 Å,c = 4.34 Å。我们的计算结果与实验值和相关的计算结果基本一致 [15] 。以PbTiO3为例,图1给出了铁电薄膜负载一层Pt(001)的三明治结构,其中铁电薄膜取为5.5层。
考虑到不同PbTiO3终端对铁电极化和物性的影响,我们分别考虑了PbTiO3以PbO终端和TiO终端两种结构分别如图1(a)、图1(b)所示。其中正方向表示铁电薄膜的极化方向是由铁电薄膜指向Pt薄膜而负方向表示极化方向背向Pt薄膜,分别用+,−号表示极化方向,如图1所示。为方便讨论,我们记PbO终端的PbTiO3为PbO/PbTiO3,而TiO终端的PbTiO3为TiO/PbTiO3。为避免slab模型中层与层之间的相互作用,真空层厚度取为15 Å。为简化计算,我们仅取1 × 1的超胞为例,其相应CO覆盖度为0.5 ML。
3. 计算结果与讨论
3.1. CO的吸附位与吸附能
一般而言,CO在金属表面存在线式、桥式、孪式和解离式四种吸附方式 [16] 。通过计算,我们发现CO在铁电薄膜负载的Pt表面只存在垂直于表面的线式吸附模式。铁电薄膜负载的Pt表面与Pt(001)的表面结构极为类似。根据对称性分析,CO在该表面的吸附位有桥位(bridge)、洞位(hollow)和顶位(top)。表一给出了CO吸附在铁电薄膜负载Pt表面的吸附能。在这里,我们定义吸附能为: ,其中
,其中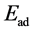 表示吸附能,
表示吸附能, 表示吸附CO之后的体系总能,
表示吸附CO之后的体系总能,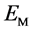 表示衬底薄膜的能量,
表示衬底薄膜的能量,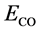 表示自由CO分子的能量。作为比较,我们在表一中也给出了CO在纯Pt(001)上的吸附能。
表示自由CO分子的能量。作为比较,我们在表一中也给出了CO在纯Pt(001)上的吸附能。

Figure 1. The optimized geometry of PbTiO3 ferroelectric thin films sandwiched by Pt monolayer: (a) for PbO terminal, (b) for TiO terminal. +, − is the ferroelectric polarization direction, the length of the arrow represents the relative magnitude of polarization
图1. 铁电薄膜PbTiO3负载单层Pt的结构模型。(a)为PbO终端,(b)为TiO终端;+,−代表铁电极化方向,箭头长短表示相对极化大小
计算得到CO在Pt(001)表面桥位、洞位和顶位的吸附能分别为−1.53 eV,−1.96 eV和−2.07 eV,该计算数值与杜森昌 [17] 的计算值一致。计算表明,CO在Pt(001)表面的最佳吸附位为桥位。为探讨极化方向、极化大小对CO吸附在Pt表面的影响,表1给出了CO在BaTiO3和PbTiO3表面负载一层Pt上的吸附能。当极化方向为负时,CO在BaO/BaTiO3负载Pt表面桥位、洞位和顶位的吸附能分别为−3.14 eV,−1.87 eV和−2.80 eV。相对Pt(001)而言,我们发现CO在洞位吸附强度稍微减弱,而在桥位和顶位的吸附强度明显增强。这主要是因为相对于纯Pt(001)表面而言,Pt薄膜的部分电荷从Pt转移到BaO/BaTiO3衬底,从而增强了CO与Pt之间的相互作用。此外,CO在BaO/BaTiO3负载Pt表面的最佳吸附位为桥位与Pt(001)表面一致。而当极化方向为正时,其相应吸附位的吸附能分别为−2.92 eV,−2.22 eV和−1.77 eV。相对于CO在Pt(001)表面的吸附能而言,CO吸附在BaO/BaTiO3洞位和桥位的吸附能仍然有所降低,但在顶位的吸附能反而提高了。对于TiO/BaTiO3终端而言,CO在洞位吸附能相对在Pt(001)表面吸附能增大,其他两个吸附位的吸附能的均有所降低。这些结果表明尽管由于极化方向均对CO的吸附能有所改变,但由于BaTiO3材料的极化强度相对较小,对CO吸附能的调控作用并不明显。
为进一步探讨极化强度对CO在Pt薄膜的吸附,我们选择了极化强度更大的PbTiO3作为衬底材料,研究了CO在PbTiO3表面负载一层Pt上的吸附,其吸附能如表1所示。以PbO/PbTiO3为例,当极化方向为负时,CO在该表面的最佳吸附位为顶位,其吸附能为−2.53 eV,相对于CO在Pt(001)表面顶位的吸附能降低了0.46 eV。而当极化方向为正时,在顶位的吸附能变为−1.39 eV,相对于CO在Pt(001)表面顶位的吸附能却提高了0.68 eV。上述计算结果表明,铁电材料的铁电极化对CO在Pt薄膜上的吸附具有很强的调控作用。这对于改良质子交换膜材料的研究具有十分重要的意义。
3.2. 机理分析
为分析铁电极化对CO在铁电薄膜负载Pt表面吸附的调控作用,我们从差分电荷密度、投影态密度两方面来分析CO与Pt的相互作用机理。作为示例,我们重点讨论CO吸附在Pt/PbTiO3这种材质表面的情况。
图2给出了Pt负载在PbTiO3表面的差分电荷密度分布。在这里,差分电荷密度表示为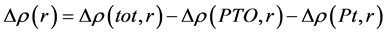 ,其中
,其中 表示Pt负载在PbTiO3表面的总电荷,
表示Pt负载在PbTiO3表面的总电荷, 表示PbTiO3衬底薄膜的电荷密度,而
表示PbTiO3衬底薄膜的电荷密度,而 则为Pt薄膜的电荷密度,PTO指PbTiO3。图2(a)给出了Pt负载在PbO/PbTiO3表面的差分电荷密度,其左端负号表示极化方向由Pt薄膜指向PbTiO3表面,而右端则由PbTiO3表面指向Pt薄膜。从差分电荷密度来看,在左端大量的电荷由Pt转移到PbTiO3表面。对于右端而言,尽管电荷同样由Pt转移到PbTiO3表面,但相对于左端要少得多。而对于TiO/PbTiO3表面来说,如图2(b)所示,其电荷转移与PbO/PbTiO3非常类似,但差别却比PbO/PbTiO3小得多。这主要是由PbO终端与TiO终端的得失电子量不同导致的。由于极化方向导致表面Pt电子环境变化不同,进而影响CO的吸附性能。
则为Pt薄膜的电荷密度,PTO指PbTiO3。图2(a)给出了Pt负载在PbO/PbTiO3表面的差分电荷密度,其左端负号表示极化方向由Pt薄膜指向PbTiO3表面,而右端则由PbTiO3表面指向Pt薄膜。从差分电荷密度来看,在左端大量的电荷由Pt转移到PbTiO3表面。对于右端而言,尽管电荷同样由Pt转移到PbTiO3表面,但相对于左端要少得多。而对于TiO/PbTiO3表面来说,如图2(b)所示,其电荷转移与PbO/PbTiO3非常类似,但差别却比PbO/PbTiO3小得多。这主要是由PbO终端与TiO终端的得失电子量不同导致的。由于极化方向导致表面Pt电子环境变化不同,进而影响CO的吸附性能。
图3给出了CO吸附在PbTiO3负载Pt表面顶位差分电荷密度分布。当极化方向由Pt薄膜指向PbTiO3薄膜时,部分电荷聚集在Pt薄膜和PbTiO3薄膜之间,从而加强了PbTiO3薄膜与Pt的耦合作用,如图3(a)、图3(c)所示;由于相互作用加强,导致了Pt薄膜与PbTiO3薄膜的距离大大缩小。然而当极化方向反向时,电荷由二者之间转移到了Pt薄膜和PbTiO3薄膜上,大大削弱了PbTiO3薄膜与Pt薄膜的耦合,从而也增加了Pt薄膜与PbTiO3薄膜之间的距离。因此,图3明显反映出由于CO的影响改变了Pt与PbTiO3的耦合。此外,我们发现当极化为正方向时,CO与Pt之间聚集的电荷比极化方向为负时要大得多,这意味着极化方向的改变可以调控CO与Pt之间的相互作用。
为进一步探讨CO与Pt/PbTiO3的相互作用,我们在图4中分别给出了CO与Pt的投影态密度

Table 1. The adsorption energy of CO adsorbed on the surface of Pt/BaTiO3 and Pt/PbTiO3
表1. CO在Pt/BaTiO3表面和Pt/PbTiO3表面的吸附能(eV)

Figure 2. The charge density difference for PbTiO3 sandwiched by Pt monolayer: (a) for PbO terminal, (b) for TiO terminal
图2. PbTiO3负载Pt的差分电荷密度分布。其中图(a)为PbO终端;(b)为TiO终端

Figure 3. The charge density difference for CO absorb on the surface of Pt/PbTiO3: (a) and (b) for the surface of Pt/PbO/PbTiO3, (c) and (d) for the surface of Pb/TiO/ PbTiO3
图3. CO吸附在Pt/PbTiO3表面的差分电荷密度分布。(a)和(b)为Pt/PbO/PbTiO3表面;(c)和(d)对应Pt/TiO/PbTiO3表面
(projected density of states)。为便于比较,我们同时也给出自由CO分子与CO分子吸附在Pt(001)表面的投影态密度。从图4(a)可以看出费米面附近,CO主要由4σ、1π、5σ 三个分子轨道组成。当CO分子吸附在Pt(001)表面后,一方面Pt的5d电子转移部分电荷给CO分子;另一方面CO反馈部分电荷到Pt的5d轨道,从而使得CO与Pt发生较强的杂化。图4(b)不仅反映出这种杂化相互作用,而且表明CO分子的4σ、1π、5σ三个分子轨道均参与了这种杂化。当加载衬底PbTiO3后,这种相互作用发生了一定的改变。当极化方向为正时,如图4(c)和图4(e)所示,三个分子轨道与Pt的杂化明显得到减弱,而当极化方向为负时三个分子轨道与Pt的杂化加强。因此,铁电极化所诱导的界面极化电场有效的调控了CO分子轨道与Pt 5d轨道之间的耦合强度,从而改变了CO分子与Pt薄膜的相互作用。
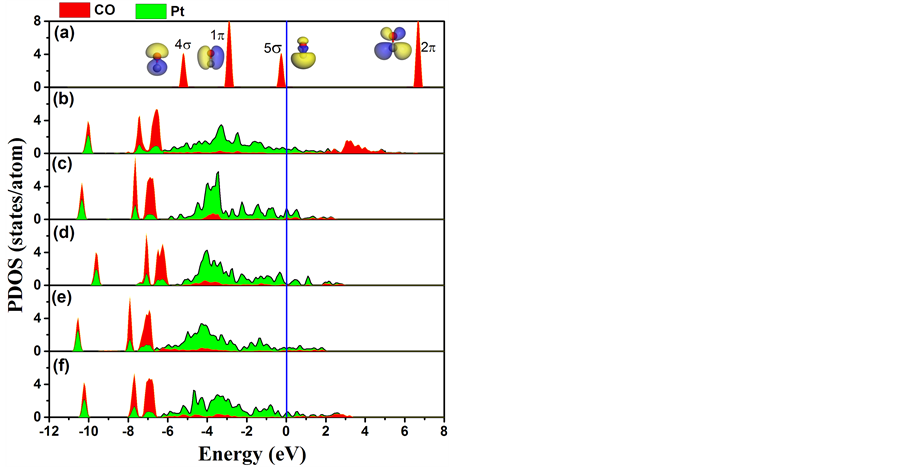
Figure 4. The PDOS: (a) for a free CO molecule; (b) for CO adsorbed on the top position of Pt (001); (c), (d) for CO adsorbed on the surface of Pt/PbO/PbTiO3 by positive or negative polarization direction; (e), (f) for CO adsorbed on the surface of Pt/TiO/PbTiO3 by positive or negative polarization direction. The Fermi level is set to 0 eV
图4. CO,Pt的PDOS,费米能级设定为0 eV。(a)为自由CO分子的PDOS,(b)为CO吸附在Pt(001)顶位的PDOS;(c),(d)为极化方向为正或负方向时CO吸附在Pt/PbO/PbTiO3表面上的PDOS;(e),(f)为极化方向为正或负方向时CO吸附在Pt/TiO/PbTiO3表面上的PDOS
4. 总结
本文采用基于密度泛函理论的第一性原理方法,分别研究了CO在铁电薄膜BaTiO3和PbTiO3负载Pt表面的吸附。计算结果表明,铁电薄膜极化的大小与方向对CO的吸附有重要影响。特别是当极化方向为负时,CO在Pt/PbO/PbTiO3表面顶位的吸附能为−2.53 eV,而当极化方向反向时,CO的吸附能变为−1.39 eV。这表明铁电薄膜的铁电极化可有效调控CO在Pt表面的吸附。通过差分电荷密度分布与PDOS分析,我们认为这种调控作用主要是通过界面电场改变CO分子轨道与Pt 5d轨道的杂化强弱实现的。这些研究结果为设计抗CO中毒的催化剂铂金材料提供了很好的铺垫。
基金项目
国家自然科学基金(批准号:11074212,11474245,11202054)和新世纪优秀人才计划(批准号:NCET-12-0722)资助的课题。