1. 引言
费–托(F-T)合成是煤间接液化的一个重要途径,是利用化石能源的有效工艺技术之一。熔铁基催化剂是第一个实现商业化的F-T合成催化剂。由于铁基催化剂F-T合成活性高,具有高水煤气变换性能,更适合于煤基合成气的F-T合成,是目前世界上很多国家以及各大石油公司竞相开发的催化剂。由于费托合成反应是一个强放热反应,所以浆态床反应器是克服这个问题的理想反应器。浆态床反应器由于受反应介质的影响,反应温度低。传统熔铁催化剂是高温(573~623 K)费–托合成反应催化剂,在低温下活性较差。本文通过研究工艺条件对Fe3O4-FeO基熔铁催化剂F-T合成反应性能的影响,得到一种低温高活性的熔铁催化剂使之适用范围更广。
2. 实验部分
催化剂制备采用电阻炉熔融法,冷却后经破碎,筛取不同粒度备用[]。F-T合成反应在微反-色谱连续流动固定床装置上进行,反应器内径
8 mm
。2 ml 的催化剂用同粒度的石英砂按1:1 的比例稀释,置于反应器的等温区。催化剂首先用纯氢还原24 h,还原温度为673~773 K, 压力为1.0 MPa,空速为10,000 h−1。还原后冷却至473 K以下切换成合成气,缓慢升温至反应温度,待达到稳态(约12 h)后,反应6~10 h 取样分析。
反应的尾气由色谱在线分析,用热导检测器(TCD)和TDX-1填充柱分析CO、CH4和CO2,气态烃采用GDX104柱和FID在线检测,收集的液相产物分离后,油相用GC/MS 5973/6890N和HP35MS毛细管柱测定,水相有机物通过GDX104柱和FID检测。
3. 结果与讨论
3.1. 反应温度的影响
图1是反应温度对F-T合成反应性能的影响,由图1可以看出,随着反应温度的升高,CO和H2转化率逐渐增加,甲烷选择性随之增加,二氧化碳选择性总的变化不大,低碳烯烷比增加明显,烃产物分布向低质烃方向移动。温度高于533 K时,CO转化率维持在90%以上。随着反应温度的升高,C2-C4和

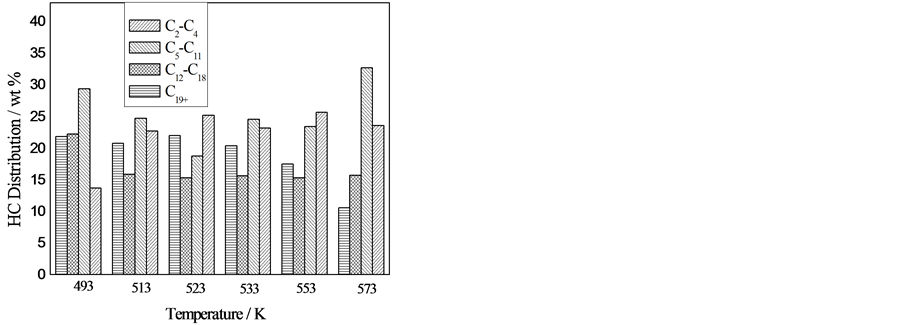 (反应条件:H2/CO = 1.5, GHSV = 3000 h−1, P = 2.0 MPa)
(反应条件:H2/CO = 1.5, GHSV = 3000 h−1, P = 2.0 MPa)
Figure 1. Effect of temperature
图1. 反应温度的影响
C5-C11烃在493 K和573 K时两者选择性较大差距,但是513 K~553 K时两者选择性接近。C12+选择性呈下降趋势逐渐明显。
由图1可以看出,随着反应温度的升高,低碳烃含量逐渐升高,且低碳烯烃的含量明显高于对应的烷烃,表明该熔铁催化剂具有较强的抑制加氢的能力。且随着温度的升高,这种抑制作用加强。在本文条件下,碳和氧平衡95%左右情况下,C1+收率为74~157 g/Nm3(合成气),C5+收率55~89 g/Nm3(合成气)。表明该熔铁催化剂在该条件下具有较好的活性,选择性以及收率。
3.2. 反应压力的影响
压力对F-T反应性能的影响如图2所示,从图2可以看出,随着反应压力的增加,CO转化率逐渐升高,CO2选择性略有下降。费托合成是体积减小的反应,增加体系总压能使反应向正向进行,故随压力增加,CO转化率逐渐增加。而WGS反应为等分子可逆反应,且F-T合成中WGS反应在接近热力学平衡的条件下进行,故总压对其平衡影响不大,但由于总压对F-T反应速率的影响大于对WGS反应速率的影响,使得总烃量的增加大于CO2量的增加,导致CO2选择性有所下降。由图2还可以看出,随着反应压力的增加,甲烷选择性变化不明显,C1-C4烃的选择性呈升高的趋势,C5-C11汽油馏分段增加,C12-C18柴油馏分段变化不大,重质烃C19+呈减少的趋势,从热力学上看,加压有利于链增长几率的增大[1] [2] ,但由于所采用的是固定床积分反应器,积分效应明显,有利于加氢链终止反应,得到的高碳烃就越少,从而导致C19+含量减少。
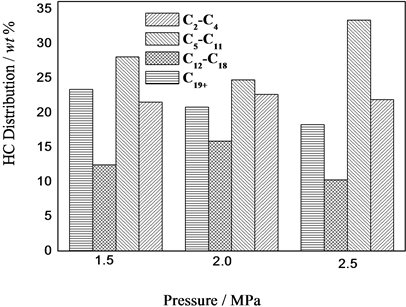 (反应条件:T = 513 K, H2/CO = 1.5, GHSV = 3000 h−1)
(反应条件:T = 513 K, H2/CO = 1.5, GHSV = 3000 h−1)
Figure 2. Effect of pressure
图2. 压力的影响
3.3. 原料气H2/CO的影响
原料气中H2/CO比对F-T反应性能的影响如图3所示。由图3可知,随着H2/CO比的增加,CO的转化率略有增加,这是因为低H2/CO比原料气有利于链增长反应,而高H2/CO比原料气有利于加氢链终止反应[3] -[5] ;由图3可见,随着氢碳比的增加,甲烷的选择性增加,而CO2的选择性降低,这是因为当反应压力不变,而原料气比增大时,反应气中的H2分压升高,有利于加氢反应,并使得WGS反应向逆方向进行[6] ;CH4选择性增加是因为合成气中H2/CO增大,体系中H2分压增加,使得催化剂活性表面富氢,阻止碳链之间的结合,加快了链的终止速率,使得CH4选择性得以增加。
由图3还可以看出,随着原料气H2/CO比的增大,低碳烃的选择性呈升高的趋势,高碳烃选择性呈下降的趋势,这是因为原料气H2/CO比增大有利于加氢链终止反应,而不利于链增长反应。由此可见,低氢碳比利于重质烃的生成,高氢碳比利于轻质烃的生成。Dry [7] 认为由于费托合成反应速率的控制步骤是强化学吸附的一氧化碳与弱的氢吸附之间的反应,因此,总反应速度主要决定于氢的分压。
3.4. 原料气空速的影响
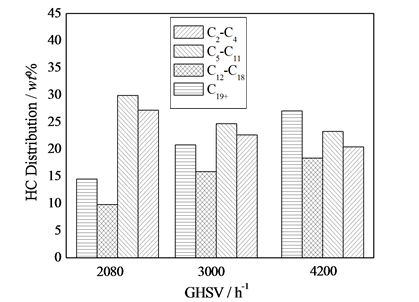
由图4可以看出,空速对F-T反应性能的影响,随着空速的升高,转化率降低。这是因为空速增加,一方面使得外扩散阻力降低有利于CO转化率的提高,另一方面,又使得原料气在床层中的线速增大而缩短了气–固之间的接触时间,不利于反应物在催化剂表面的停留,从而导致CO转化率降低。在本实验低温低空速条件下,后一种情况占主导[8] ,所以转化率降低;随着空速(2080~3000 h−1)升高,甲烷选择性升高,空速继续升高至4000 h−1,甲烷选择性几乎不变,随着空速(2080~3000 h−1)升高,二氧化碳选择

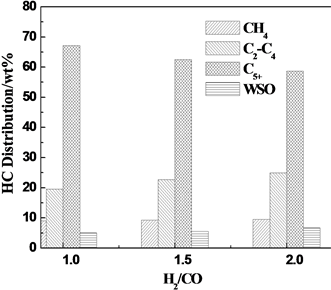 (反应条件:T = 513 K, P = 2.0 MPa, GHSV = 3000 h−1)
(反应条件:T = 513 K, P = 2.0 MPa, GHSV = 3000 h−1)
Figure 3. Effect of H2/CO
图3. 原料气H2/CO的影响
 (反应条件:T = 513 K, P = 2.0 MPa, GHSV = 3000 h−1)
(反应条件:T = 513 K, P = 2.0 MPa, GHSV = 3000 h−1)
Figure 4. Effect of space velocity
图4. 空速的影响
性升高,3000~4000 h−1二氧化碳选择性降低。这是因为低温低空速下,热力学上有利于WGS反应,而空速的升高,提供了较多的H2O参与反应,故CO2选择性升高,但同时也要考虑到热力学平衡推动力等问题,所以二氧化碳选择性较复杂。
由图4还可以看出,随着空速的升高,低碳烃的含量逐渐减少,高碳烃的含量逐渐增加。低温低空速下,随着空速的升高,外扩散阻力降低有利于低碳烃的逃逸。从理论上讲,随空速升高,C1-C4烃的选择性应升高,C5+选择性应降低;但是,由于所采用的是固定床积分反应器,积分效应严重,而导致相反的结果。由原料气H2/CO的影响讨论可知,在一定的空速下H2/CO比越高,CO转化率亦越高,生成的低碳烃亦越多;随着反应气在催化剂床层的下移,H2/CO比逐渐增大,尤其在低空速下,积分效应尤其明显,尾气中的H2/CO比迅速增大,使得催化剂床层后部微分转化率升高,低碳烃的含量增加。
3.5. 催化剂粒度的影响
由图5不同粒度催化剂对F-T反应性能影响可以看出,随着粒度减小,CO的转化率增加,直到0.075~0.1 mm以后CO转化率基本保持不变,这是因为消除了催化剂的内扩散影响[9] [10] ;随着催化剂粒度的减小,0.15~0.25 mm和0.1~0.15 mm时,内扩散还没有消除时转化率增加,转化率增加,这可能是因为粒度减小,在相同时间内催化剂上的氢分压增大,则甲烷的选择性增加,颗粒小于0.075~0.1 mm内扩散已基本消除,此时转化率已经基本不变,此时粒度减小,则氢分压所占比例变化不大,则对甲烷的选择性影响也不明显;随着粒度的减小,低碳烃选择性增加,高碳烃选择性减小,这可能是因为随着粒度减小,内扩散慢慢消除,使得低碳烃易于逃逸,不利于碳链的增长,小于0.075~0.1 mm范围F-T反应产物的烃分布几乎是一致的,认为内扩散已经消除;由图5可以看出随着粒度减小,C2+和C5+收率也是相应的增加,小于0.075~0.1 mm范围收率基本不变,可以认为此后内扩散已经消除。
4. 结论
1) 反应条件对Fe3O4-FeO基熔铁催化剂F-T合成反应性能影响规律和其它铁基催化剂F-T合成类似。
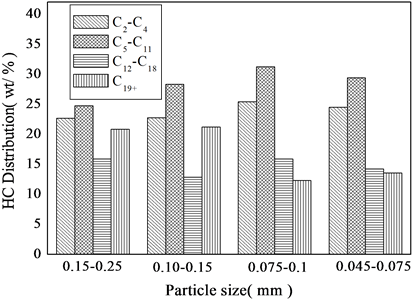
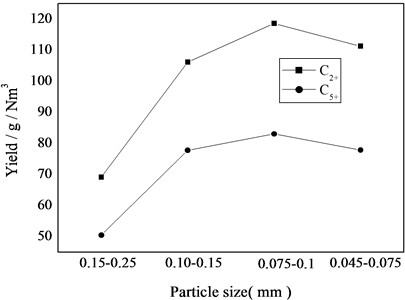 (反应条件:T = 513 K, H2/CO = 1.5, P = 2.0 MPa, GHSV = 3000 h−1)
(反应条件:T = 513 K, H2/CO = 1.5, P = 2.0 MPa, GHSV = 3000 h−1)
Figure 5. Effect of particle size
图5. 催化剂粒度的影响
2) 与传统熔铁催化剂相比该熔铁催化剂在低温下对F-T合成反应有较高的活性和总有效烃选择性,493~573 K,CO转化率为52% ~ 95%左右,烃收率74 ~ 157 g/Nm3(合成气)。Sasol工业化的低温F-T合成催化剂F-T活性为493K时合成气转化率46%,甲烷,汽油和硬蜡选择性分别是7%,14%,27%。与Sasol工业化的低温F-T合成催化剂F-T活性[7] 相比,该催化剂适用于低温费–托合成反应工艺,且对浆态床工艺有潜在的应用开发前景。
致谢
感谢浙江省教育厅项目(Y201326527)对本工作的支持。