摘要:
目的:建立顶空气相色谱法检验氯苯达诺盐酸盐原料药中残留溶剂的方法。方法:采用6%氰丙基苯基-94%二甲基聚硅氧烷为固定液的毛细管柱(DB-624, 0.53 mm × 30 m, 3.0 μm),程序升温,初始温度40℃,保持3 min,以20℃/min的速率升温至240℃,保持3 min;氢火焰离子化检测器(FID),检测器温度250℃,进样口温度200℃,载气为氦气,流速5 mL/min,进样体积1 mL。分流比1:1。结果:5种有机溶剂(甲醇、乙醇、乙腈、苯、甲苯)进样浓度与峰面积呈良好线性关系,相关系数均大于0.9990,加样回收率在96.0%~103.0%之间。结论:此法准确可靠,灵敏度高,可用于氯苯达诺中残留溶剂的测定。
Abstract:
Objective: To establish a GC method for the determination of residual solvent in clofedanol. Methods: DB-624 chromatographic column (coated with 6% cyanopropylphenyl-94% dimethylpolysiloxane, length 30 m, I.D. 0.53 mm, film 3.0 μm) was adopted. The column temperature was programmed, kept at 40˚C for 3 min, and then increased by 20˚C/min to 240˚C for 3 min. The temperature of FID detector was 250˚C and the temperature of inlet was 200˚C. The carrier gas was He at a flow rate of 5 mL/min. The injection volume was 1 mL. Results: The calibration curves of five solvents all showed good linearity in a detected concentration range (r > 0.9990); the range of average recovery was between 96.0% and 103.0%. Conclusion: The method was accurate, reliable and sensitive, and can be used for assay of residual organic solvent in clofedanol.
1. 引言
氯苯达诺除有中枢性镇咳作用外,还有抗组织胺作用和阿托品样作用,能减轻支气管痉挛和粘膜充血性水肿。适用于呼吸道急性感染引起的干咳或阵咳,常与祛痰药合用。在氯苯达诺的合成过程中用的溶剂有苯、甲苯、甲醇、乙醇、乙腈,这些溶剂在成品中不可避免的会残留,根据中国药典及ICH (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use),人用药品注册技术要求国际协调会)对溶剂残留有限度规定,故本文研究了测定氯苯达诺残留溶剂含量的方法。
2. 仪器与试药
Agilent 7890N气相色谱仪(美国安捷伦);Agilent 7694E顶空进样器(美国安捷伦);毛细管柱:DB-624 (30 m × 0.53 mm, 3.0 μm);苯、甲苯、无水甲醇、乙醇、乙腈均为分析纯,N,N-二甲基甲酰胺(以下简称DMF)为色谱纯;氯苯达诺(生产单位为江苏宝众宝达药业有限公司,批号:1041210001、1041210002、1041210003)。
3. 方法与结果
3.1. 溶液制备
对照储备溶液制备:精密称取乙腈205 mg,甲苯445 mg,无水甲醇1500 mg,无水乙醇2500 mg,苯溶液(1 mg/mL) 1.0 mL,置于100 mL容量瓶中,用DMF水溶液(10→100)稀释至刻度,摇匀。
对照溶液制备:精密移取1.0 mL对照储备溶液于100 mL容量瓶中,加DMF水溶液(10→100),稀释至刻度,摇匀即得,精密移取10.0 mL置于顶空瓶中。
供试品溶液制备:取氯苯达诺约0.5 g,精密称定,加DMF水溶液(10→100) 10 mL,溶解,作为供试品溶液。
3.2. 色谱条件
DB-624毛细管柱,FID (火焰离子化,Flame ionization Detector)检测器,检测器温度250℃,进样口温度200℃;载气为氦气,纯度99.99%,流速5 mL/min;采用程序升温,初始温度40℃,保持3 min,以20℃/min的速率升温至240℃,保持3 min;顶空瓶平衡温度80℃,平衡时间25 min,进样量1 mL;定量环温度120℃,传输线温度130℃ [1] 。
3.3. 系统适用性与精密度试验
记录色谱图,5种溶剂分离度良好,见图1,经验证出峰顺序为甲醇、乙醇、乙腈、苯、甲苯;各峰之间的分离度依次为2.2、10.6、10.6、12.0、8.1,分离度均大于1.5,色谱峰完全分离;其峰面积的相对标准偏差(RSD)分别为4.4%、4.5%、3.7%、2.5%、8.1%,峰保留时间的相对标准偏差(RSD)分别为0.02%、0.01%、0.01%、0.01%、0.02%,表明仪器精密度良好,方法重现性好。
3.4. 线性关系及检测限试验
分别精密量取对照品贮备液200 μl,150 μl,100 μl,50 μl,10 μl,5 μl,置于顶空进样瓶中,再分别精密量取10 mL DMF水溶液(10→100),混匀,按3.2项下的色谱条件进样,记录色谱图,以浓度(C)对峰面积(A)进行线性回归,结果见表1。
通过逐级稀释降低浓度,进样测定,测得甲醇、乙醇、乙腈、苯、甲苯的检测限分别为0.171 μg/mL、0.425 μg/mL、0.0699 μg/mL、0.0127 μg/mL、0.00585 μg/mL,折算成样品中的含量分别为3.4 μg/g,8.5 μg/g,1.4 μg/g,0.03 μg/g,0.12 μg/g。
3.5. 回收率
分别各配制测定限度的50%,100%,150%加入对照溶液的样品溶液,按3.2项下的色谱条件,按外标法计算,甲醇、乙醇、乙腈、苯、甲苯平均回收率分别为98.8%、99.6%、100.7%、101.8%、100.5%,RSD分别为5.3%、6.5%、2.8%、9.4%、7.2%。
3.6. 重复性试验
取同一批供试品(批号:1041210001),共6份,按3.1项的方法制备供试品溶液,按3.2色谱条件进样,记录色谱图,按外标法计算样品中甲醇、乙醇、乙腈、苯、甲苯的残留量分别为290 μg/g、89 μg/g、未检出、未检出、5 μg/g,RSD分别为5.6%、4.2%、未检出、未检出、5.7%。
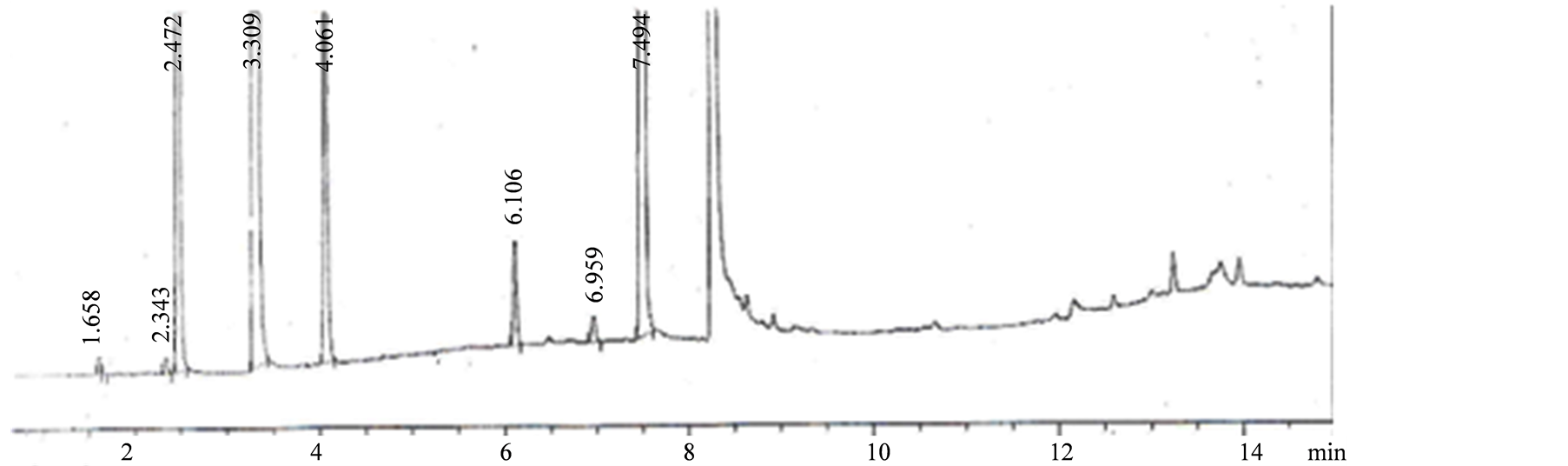 1:甲醇(2.4 min),2:乙醇(3.3 min),3:乙腈(4.0 min),4:苯(6.1 min),5:甲苯(7.4 min)
1:甲醇(2.4 min),2:乙醇(3.3 min),3:乙腈(4.0 min),4:苯(6.1 min),5:甲苯(7.4 min)
Figure 1. Reference substance chromatogram
图1. 对照品色谱图

Table 1. Linear regression equation and linear range determination results
表1. 线性回归方程和线性范围测定结果

Table 2. Sample determination results
表2. 样品测定结果
3.7. 样品测定
取氯苯达诺3批(批号:1041210001、1041210002、1041210003),按3.1项的方法制备供试品溶液,按3.2色谱条件进样,记录色谱图,按外标法计算样品中各溶剂的残留量,3批样品的测定结果见表2。
4. 小结
4.1. 平衡温度的选择
通常较高的平衡温度可提高灵敏度,减少平衡时间,但通过高温度会导致供试品及残留溶剂分解或发生其他变化,还可引起顶空的耐压和气密性等问题,增大分析误差。本文预试一系列温度,80℃比较适宜[2] 。
4.2. 平衡时间的选择
考察了20 min,25 min,30 min,40 min等不同平衡时间对结果的影响,表明了30 min后峰面积基本不再增大,故选择30 min为平衡时间。