1. 引言
高级氧化技术能够产生活性极强的自由基,将难降解有机物氧化分解成小分子物质,甚至直接矿化为C2O和H2O。高级氧化技术主要包括UV/H2O2、臭氧氧化、芬顿试剂法、光催化氧化、超声催化氧化法、超临界水氧化法等。在众多高级氧化技术中,光催化技术因其具有选择性小、矿化度高、无二次污染、操作条件容易控制、尤其可以利用太阳光作为光源,尤为引起人们的关注。
但光催化剂失效及再生问题是光催化技术实际应用中的一个主要问题,所以针对该方面的研究很有意义。但与研究提高光催化剂催化效率、提高光催化剂可见光活性的文献相比,研究光催化剂失效以及再生的文献却相对较少[1] 。本文对国内外文献中关于失效光催化剂的再生方法划分为煅烧、清洗、氧化、树脂吸附四种方法,针对各种再生方法的再生效果及技术关键进行了综述。
2. 再生方法
2.1. 煅烧
煅烧再生方法是一种常见的光催化剂再生方法,可以在较高的温度下将导致光催化剂失效的吸附在光催化剂表面的反应中间产物等燃烧掉,也经常应用于其他催化剂的再生中。煅烧再生方法的关键是选取合适的煅烧温度。Joana T. C. [2] 采用Hombikat UV100和H600、S450三种光催化剂降解环己烷,以400℃在空气中煅烧失效的光催化剂Hombikat UV100和H600,发现由于表面吸附了热稳定性很强的碳酸盐和羧酸盐,导致煅烧再生无效,但同样再生煅烧温度下就能够再生S450,发现是由于S450表面的污染物与其他光催化剂不同,且该污染物热稳定性较低。所以必煅烧再生。
Cao L.等 [3] 针对降解甲苯后失效的光催化剂采用程序升温氧化技术(TPO)分析失效光催化剂的表面,失效光催化剂表面有三个峰,分别为280℃、360℃、和420℃,说明有不同的碳化合物存在于失效光催化剂表面,并且420℃能够将所有的碳化合物燃烧干净。然后对失效光催化剂采用350℃和420℃两种煅烧温度进行煅烧再生试验,试验发现350℃的煅烧温度下只能实现光催化剂的部分再生,而420℃的煅烧温度下能够实现光催化剂的完全再生。该试验结果与TPO技术分析结果一致。
Raquel P.等 [4] 采用TiO2/M-MCM-41(M=Cr or Ce)在UV-A和可见光下降解H2S,由于Cr6+还原为Cr3+,并且硫化物沉积在光催化剂表面占领活性位导致光催化剂失效。针对失效的光催化剂20%TiO2/ Cr-MCM-41采用TG-MS分析,发现硫化物(SO、SO2)在460℃具有最大值,说明通过460℃煅烧能够将硫化物分解。
Sujaree K.等 [5] 采用自制的光催化剂在可见光下降解有机染料甲基橙,失效后的光催化剂采用煅烧的方法再生。由于煅烧温度高于250℃会影响光催化剂的可见光活性,且煅烧高于350℃会导致N-甲基吡咯烷酮烧结,使比表面积下降、颗粒尺寸增大,所以试验采用了200℃和250℃进行再生,但试验结果发现不能完全恢复光催化活性。该试验结果与TGA分析结果相吻合,因为TGA分析发现碳化合物沉积在光催化剂表面导致光催化剂失效,并且需要420℃以上才能够去除沉积碳,所以200℃和250℃的煅烧温度过低导致煅烧再生不能完全恢复光催化活性。
但由于过高的煅烧温度会导致光催化剂晶型从锐钛矿变为金红石,并使纳米TiO2烧结聚集 [3] ,所以在一些制备光催化剂再生过程中,会有一些研究者采用制备温度进行再生 [3] [6] 。如Cao L.等 [3] 针对失效的TiB和TiC分别采用制备温度350℃和420℃在空气中煅烧2 h进行再生,但发现350℃的煅烧温度过低,没有实现完全再生。Jorge M.等 [6] 针对失效的光催化剂采用制备温度450℃在空气中煅烧3 h进行再生,在第一次再生后,光催化活性基本完全恢复,说明煅烧温度比较适宜,但在第二次煅烧再生后,光催化活性略有降低,作者认为可能是沉积在光催化剂表面的碳没有完全去除,或者煅烧对光催化剂表面性质产生了影响。
确定煅烧温度时可采用TPO或TGA分析技术,确定去除光催化剂表面的污染物所需的温度,并考虑煅烧温度对光催化剂表面性质的影响,结合试验分析最终确定合理的煅烧温度。如果只有较高的煅烧温度才能去掉光催化剂表面的污染物,而较高的煅烧温度对光催化剂表面性质影响较大,降低光催化剂活性,则不适宜采用煅烧方法进行再生。
2.2. 清洗
清洗也是一种常用的光催化剂再生方法,通过清洗将吸附在光催化剂表面的污染物去除。清洗方法包括单纯水洗,超声清洗以及采用清洗液进行清洗,如碱洗,有机溶剂清洗等。清洗再生相对比较简单,易于操作。
选择清洗方法时关键需要考虑光催化剂表面的污染物性质,如在Seree T.等 [7] 采用TiO2降解Cr(VI)的试验中,由于Cr(OH)3吸附在光催化剂表面导致光催化剂失效,所以选用NaOH溶液进行清洗,并考察了NaOH溶液浓度对清洗效果的影响,发现3M NaOH的清洗效果最好,能够恢复光催化剂活性。Sun R.等 [1] 采用TiO2薄膜降解八甲基三硅氧烷的试验中,SiOx沉积在TiO2薄膜表面导致光催化剂失效,发现采用pH > 12的NaOH溶液处理约20 min能够完全去除表面的SiOx,再生后催化活性与新TiO2薄膜相当。
降解不同目标物的光催化剂在清洗再生过程中,由于污染物在光催化剂表面的吸附强度不同,再生效果也会有所不同。Alexandre V. V.等人 [8] 采用负载型TiO2降解二乙基硫醚过程中,失效的光催化剂采用直接清水清洗能够完全恢复活性。但Gandhi V. G. [9] 采用TiO2降解邻苯二甲酸的过程中,失效的光催化剂采用清水清洗与甲醇清洗相结合的方式,仍然没有取得较好的再生效果,主要是由于光催化剂表面吸附的污染物具有很强的吸附性,较难通过清洗去除。
超声波清洗再生是一种很有效的清洗再生方式,适合于纳米光催化剂的再生,能够加强清洗的效果。Jing L.等 [10] 在采用纳米ZnO和TiO2降解n-C7H16和SO2的试验中,采用去离子水超声波清洗,失效的光催化剂基本完全恢复了活性。超声波清洗还可以与清洗液清洗相结合,Yang W. [11] 等采用TiO2-rGO/ ASC光催化剂在可见光下去除NO,在对失效的光催化剂采用超声波加氨水清洗再生,新光催化剂的NO转化率为71.89%,超声波加氨水清洗再生后NO转化率为63.72%,再生效果优于热再生、超声水洗、和热蒸汽再生。
可见,由于光催化剂表面的污染物质不同,所以针对不同的失效光催化剂最佳清洗方法也会有所不同。另外,超声波辅助清洗与清洗剂清洗相结合能够提高清洗效果,是一种较为有效的清洗再生方式。
2.3. 氧化法
采用氧化的方法去除光催化剂表面的污染物,进而恢复光催化剂的活性,统称为氧化法。
氧化法中紫外照射的方法比较常用,该方法可以直接在反应器内进行,比较方便易行。如Jeong M. [12] 采用TiO2降解甲苯,针对失效的光催化剂在70%相对湿度下采用UV照射2000 min,发现再生后的光催化剂光催化活性完全恢复。Li X.等 [13] 采用TiO2降解H2S,光催化剂失效后采用波长为185 nm的臭氧灯照射48 h,发现光催化剂完全恢复活性。Thevenet F.等 [14] 采用负载型TiO2降解乙炔,失效的光催化剂通过紫外照射再生80%吸附在光催化剂表面的酸矿化为CO2,实现光催化剂的再生。Jeong J.等 [15] 采用负载型TiO2降解甲苯,光催化剂失效后,采用五种方法进行再生,对比再生结果。再生方法分别为开紫外灯通入潮湿空气,开紫外灯通入干燥空气,开紫外灯通入潮湿氮气,关紫外灯通入干燥含有臭氧的空气,关紫外灯通入潮湿空气。试验结果表明:开紫外灯通入潮湿空气和干燥空气以及关紫外灯通入臭氧均取得了很好的再生效果,但关紫外灯通入潮湿空气和开紫外灯通入潮湿氮气的再生效果不佳。
还可以通过加入氧化剂的方法实现失效光催化剂的氧化再生。最常加入的氧化剂是H2O2,Gandhi V. G.等 [9] 的试验中,采用TiO2降解邻苯二甲酸,1 g失效的光催化剂用10 ml 30% H2O2搅拌1 h进行再生,实现了光催化剂完全再生,活性与新鲜的光催化剂相同。也可以采用H2O2与紫外照射相结合的方法,如Miranda-Garcia N.等 [16] 采用负载型TiO2降解15种水中难降解污染物,针对失效的光催化剂采用1 M H2O2与紫外照射相结合的方式,照射时间为8 h,与3 M NaOH清洗1 h、3 M NH4OH清洗1 h、热处理400℃ 180 min相比,H2O2与紫外照射相结合的再生方法的再生效果最佳。
臭氧也是一种较常加入的氧化剂,Jeong J.等 [15] 采用负载型光催化剂降解甲苯,加入臭氧对失效的光催化剂进行再生,发现臭氧能够有效降解TiO2表面的中间产物,实现光催化剂的再生。
2.4. 树脂吸附
Li Y.等 [17] 采用纳米ZnO降解甲基橙,失效的光催化剂和离子交换树脂和水混合一定时间,通过以下反应实现光催化剂的再生:

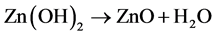
沉积在光催化剂表面的无机沉淀通过以下反应被去除:

水中的可溶Zn2+通过离子交换反应回收利用:

试验结果表明,离子交换树脂吸附法能够将失效的光催化剂完全再生。
3. 再生方法的可重复性问题
有效的再生方法应该具有较好的可重复性,即在每次再生后都保持较高的光催化活性恢复率。一些研究者也针对再生方法的可重复性问题进行了研究。
Yang W. [11] 等采用TiO2-rGO/ASC光催化剂在可见光下去除NO,新的光催化剂NO转化率为71.89%。失效后的光催化剂分别经热再生、超声水洗、超声氨水清洗、热蒸汽再生四种再生方法再生,再生后NO转化率分别为52.42%、59.95%、63.72%、62.23%。其中热蒸汽再生方法成本较低且较为有效,所以进一步考察热蒸汽再生方法的再生次数对再生效果的影响,发现NO转化率随再生次数增加分别为62.23%、58.87%、54.23%、55.72%、54.02%。可见在前三次再生后,NO转化率均略有下降,但在第四次和第五次再生后,NO转化率基本稳定在55%左右,说明该再生方法具有一定的稳定性和可重复性。
Shavisi Y. [18] 等采用TiO2/LECA光催化剂降解污水中的工业污染物,光催化剂失效后按照水洗、曝气清洗、3 g/L NaCl溶液浸泡3 h、250℃热处理30 min四个步骤进行再生。试验考察了再生次数对光催化剂活性的影响,试验共再生3次,发现每次再生后光催化活性都会降低14%左右,新的光催化剂氨氮去除率接近100%,但再生3次后,氨氮去除率低于60%。分析认为再生后光催化剂表面吸附能力的降低和活性位的减少是导致光催化活性降解的主要原因,也说明该种再生方法的可重复性较差。
由以上的研究可见,确定一种有效的再生方法时,考察再生方法的可重复性与考察再生方法的光催化活性恢复率同样重要,但国内外文献中关于光催化剂再生方法的研究中,对再生方法的可重复性的研究相对较少,应该在以后的研究中加以重视,不仅对比研究各种再生方法的再生效果,也应对比研究各种再生方法的可重复性问题。
4. 总结
综述了国内外文献中关于失效光催化剂的再生方法以及再生效果。煅烧再生方法最重要的是选取合适的煅烧温度,如果温度过低,光催化剂表面的污染物没有被去除,再生效果较差,但温度过高,又会导致光催化剂晶型的变化以及纳米TiO2烧结聚集,影响表面性质。清洗再生方法最重要的是要结合污染物的性质选取合适的清洗剂,超声清洗也是一种有效的清洗方法。氧化再生方法可以考虑紫外照射与加入氧化剂相结合,提高再生效果。
在考察再生方法的再生效果同时,再生方法的可重复性也同样重要,但相关研究相对较少,在之后的研究中应该加以重视。
致谢
本文由国家自然科学基金(51208172)和中国博士后基金(2014M551496)资助。