1. 引言
干旱是影响植物生长和作物产量的重要逆境因子[1] [2] 。据估计,全球有45%以上的农业用地受到干旱的影响[3] ,农作物的产量和收成急剧减少。干旱影响了植物代谢和生长发育,引起植物体内发生一系列的生理生化反应,严重时可抑制植物生产和降低产量。干旱胁迫对作物的影响是多方面的,可以表现在形态、生理、生化、产量等各个方面。很多研究者就作物的抗旱性问题进行了比较深入的探索,得到了较好的研究成果,对玉米[4] 、水稻[5] 、大豆[6] 、小麦[7] [8] 、花生[9] 、甘薯[10] 等作物进行了比较全面的抗旱性研究。在抗旱性研究中所建立的干旱条件多是人工控制的干旱或采用PEG渗透调节的方式,在温室、培养箱或其他模拟环境中进行[11] -[13] 。但在油菜抗旱性研究中,多是采用PEG模拟干旱的方式进行的,而在人工控制的干旱条件下进行的研究相对较少。
甘蓝型油菜(Brassica napus L.)属十字花科芸薹属植物,是世界上最重要的油料作物之一,也是中国第一大油料作物。但是,我国油菜生产常因春旱和秋冬旱出现出苗不齐、出叶较慢、植株矮小等情况,导致油菜产量及品质下降[14] [15] ,从而限制了油菜的生产和产业发展。IrrE基因是来源于耐辐射奇球菌(Deinococcus radiodurans)的全局性调控因子,参与了基因表达调控过程,与胁迫响应、能量代谢、转录调控、信号传递等密切相关[16] 。我们前期的研究表明,IrrE基因在大肠杆菌中异源表达能够提高菌株的辐射抗性以及氧自由基清除能力,提高了大肠杆菌的抗盐胁迫、抗氧化胁迫以及抗高温胁迫的能力;将IrrE基因转入油菜中,结果显示转基因油菜植株能够在350 mmo/L NaCl处理下6周后正常开花结实,而对照非转基因油菜植株在相同处理2周后死亡[17] 。本研究以转IrrE基因油菜T3代植株为试验材料,以非转基因油菜植株为对照,通过自然干旱的方式处理0、14、21、28和35 d,然后复水,探讨在自然干旱胁迫下IrrE基因过表达对油菜植株相关生理生化的影响,为IrrE基因在油菜耐旱中的研究奠定基础。
2. 材料和方法
2.1. 材料
试验采用盆栽土培方式,地点为四川绵阳西南科技大学科技园。试用土壤取自西南科技大学农学试验基地,土壤类型为黄壤土。供试植物油菜种子为本实验室栽培保存的转IrrE甘蓝型油菜和非转基因甘蓝型油菜(波里马细胞质雄性不育恢复系84100-18,由四川大学遗传实验室提供)。
2.2. 材料处理与培养
选取籽粒饱满、大小均匀、无病虫害的转IrrE基因油菜种子,用75%乙醇浸泡30 s,2%的NaClO溶液浸泡8~10 min,无菌水冲洗5~6次。吸胀12 h后,置于25℃光照培养箱中萌发24 h,再用蒸馏水清洗萌发的种子2~3次。将萌发一致的种子插播在装有已灭菌石英砂的瓷盘中,浇上适量的1/4 Hoagland营养液,置于组织培养室培养(25 ± 1)℃,光照12 h,每天补充营养液2次。待幼苗长到四叶期,提取叶片DNA进行常规PCR鉴定和实时定量PCR检测,用鉴定为阳性的植株进行后续实验。实验时,在各个花盆中装入等量的营养土(腐殖土:大田土 = 2:1),选择生长良好的,长势一致的1棵非转基因植株与1棵已鉴定为转基因的阳性植株移栽到同一个花盆中,共30次重复。常规管理一段时间,使其在自然条件下正常生长,待长到6~8叶时,统一浇足水分,以后不再浇水,并移至温室中。观察两组实验中幼苗的生长情况,并在干旱的不同时期用RR-7730M土壤湿度计进行土壤含水量的测定及采取叶片进行相关指标的测定[18] 。
2.3. 叶片相对含水量的测定
叶片相对含水量(RWC)的测定采用鲜重法[19] ,称取植物叶样,精确称量鲜重Wf,将叶样浸没在水中24 h后,擦干叶片表面水分,精确称量饱和重Wt,最后将叶样在105℃杀青15 min,80℃烘至恒重,精确称量得到干重Wd。叶片相对含水量用以下公式计算:叶片相对含水量(%) = (Wf − Wd)/(Wt − Wd) × 100%。
2.4. 叶绿素含量测定
采用混合液(丙酮:无水乙醇 = 2:1)浸提法提取叶绿素[20] ,称取0.1 g植物叶片,剪成细丝后,放入加有20 ml混合液的三角瓶中,密封,4℃避光放置,直至叶片完全泛白。以混合液为对照,测定0D663和OD645,用以下公式计算叶绿素含量:叶绿素总含量(mg/g) = (20.2 × A645 + 8.02 × A663)V/1000 × W,其中V为提取液总体积;W为叶片鲜重。
2.5. 叶片中可溶性蛋白质含量的测定
可溶性蛋白采用考马斯亮蓝G250法测定[21] 。
2.6. 叶片丙二醛含量的测定
丙二醛(MDA)含量采用硫代巴比妥酸法测定[21] 。
2.7. 粗酶液的提取
称取2 g植物叶片,置于预冷的研钵中,加入少量的石英砂和预冷的4 mL 50 mmol/L磷酸缓冲液(pH值7.0,含1% PVP、0.1%巯基乙醇)于冰浴上研磨成匀浆,全部转入5 mL干净的离心管中,于4℃、13,000×g离心10 min,将上清液分装至干净的1.5 mL离心管中,保存于−20℃备用。
2.8. 抗氧化酶活性的检测
过氧化物酶(peroxidase, POD)活性采用愈创木酚法[9] ,以每分钟OD470变化0.01为一个酶活性单位(U/(g∙protein∙min));超氧化物歧化酶(superoxide dismutase, SOD)活性检测采用氮蓝四唑(NBT)法[10] ,以抑制NBT光还原50%作为一个酶活性单位(U/(g∙protein∙min));过氧化氢酶(Catalase, CAT)活性检测采用H2O2法[11] [12] ,以25℃下100 s内在反应体系中分解50% H2O2的酶量作为一个酶活性单位(U/(g∙protein∙min))。
3. 结果与分析
3.1. 转IrrE基因阳性植株的鉴定
用质粒抽提试剂盒提取大肠杆菌(Escherichia coli) IrrE-DH5α中的质粒DNA作为PCR扩增的阳性对照,以非转基因油菜的DNA为阴性对照。扩增反应中的引物为:上游CAACGTCATCCTCATCAACTC;下游TCCACCTCCGCCTTCATTT。扩增的目的片段大小为398 bp。扩增程序为:94℃变性2 min,94℃ 30 s,58℃ 30 s,72℃ 30 s,共35个循环。扩增得到的PCR产物用0.8%琼脂糖凝胶电泳检测(图1)。与质粒DNA有相同条带的转基因植株为阳性植株,可用于后续实验。
3.2. 自然干旱胁迫下叶片相对含水量的变化
在0~35 d的自然干旱条件下,土壤的含水量逐渐下降,从85.23%下降到36.22%。与土壤含水量变化一样,转基因与非转基因油菜的含水量也逐渐下降,他们分别从93.17%、92.20%下降到33.19%和26.31% (图2)。当在35 d恢复供水,土壤含水量几乎达到100%,恢复供水后第7 d和第14 d后土壤含水量分别为79.83%和72.34%,而转基因与非转基因油菜植株的叶片也逐渐恢复活力,其含水量也相应升高。但在0~35 d的干旱过程中和恢复供水后,转基因植株的含水量都略高于非转基因植株,说明在干旱胁迫下IrrE基因能增强油菜植株的叶片含水量。
叶绿素是植物光合作用的主要物质基础,其含量变化也是对逆境的一个反应指标。如图3所示,在0~35 d的自然干旱条件下,油菜植株的叶绿素含量都逐渐增大,但在同一胁迫时期内,转基因油菜的叶绿素含量比非转基因油菜的含量高。当干旱到第35 d时,油菜植株处于终极状态,叶片萎焉严重,叶片中含水量降低到极致,故而叶绿素含量较高;当地35 d给植株恢复供水后,植株的含水量逐渐增加,叶片萎焉状态得到改善,生理功能逐渐恢复,故叶绿素含量呈下降趋势,直至正常的叶绿素含量水平;但恢复过程中,转基因油菜植株的恢复能力要强于非转基因油菜。
3.3. 干旱胁迫对MDA含量的影响
干旱胁迫下,植物的细胞膜结构遭到破坏,MDA含量升高,MDA的积累会对膜和细胞造成进一步的伤害,其含量的高低反应了逆境条件下植物细胞膜的受损程度,MDA含量越高,说明植株受到的伤害越大[22] 。随着干旱胁迫时间的延长,两者MDA含量均呈上升趋势,非转基因植株MDA上升幅度大于转基因植株。恢复供水后,胁迫解除,MDA含量均下降(图4)。这说明了在自然干旱条件下,转基因油菜植株的细胞膜受损程度低于非转基因植株。
 注:M为DL2000 marker;W为水;1为质粒;2为转基因植株;3为非转基因植株
注:M为DL2000 marker;W为水;1为质粒;2为转基因植株;3为非转基因植株
Figure 1. PCR identification in transgenic Brassica napus L.
图1. 转基因油菜植株PCR检测
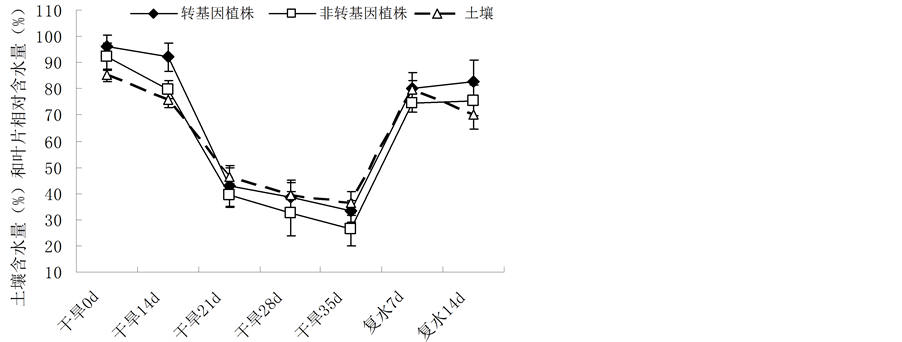
Figure 2. Change of the relative water content in the leaves of Brassica napus L. during drought stress and after restoration of water supply
图2. 在干旱胁迫和胁迫后恢复供水后植株叶片含水量的变化
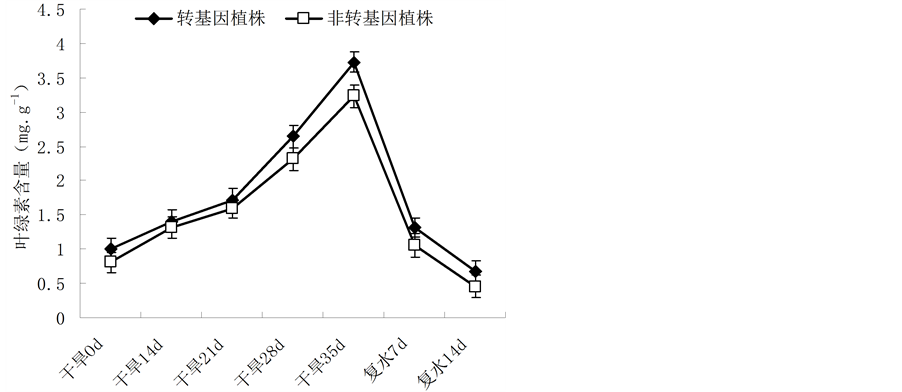
Figure 3. Change of the chlorophyll content in leaves of Brassica napus L. during different periods of drought stress
图3. 不同胁迫时间内植株叶绿素含量差异
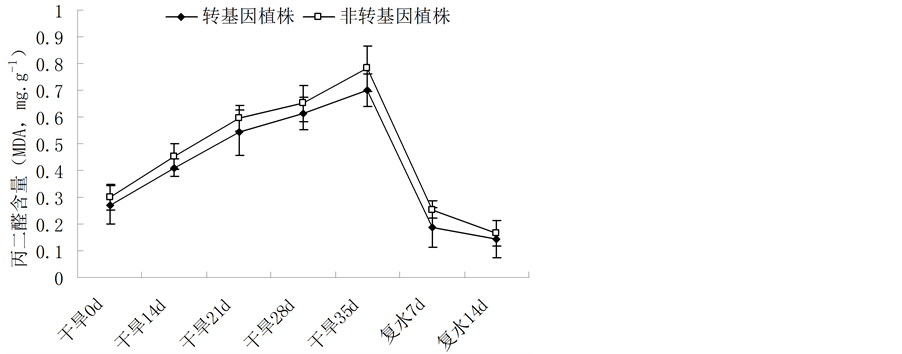
Figure 4. Change of the MDA content in leaves of Brassica napus L. during different periods of drought stress
图4. 不同胁迫时间内植株MDA含量的变化
3.4. 干旱胁迫对可溶性蛋白含量的影响
可溶性蛋白是植物体内重要的渗透调节物质之一,起到一定的渗透调节作用。其含量变化可以反映植株受到外界非生物胁迫和病虫害等生物胁迫时细胞内蛋白质合成、变性及降解等多方面的信息,因此测定其含量是了解植物抗逆性的一个重要指标。干旱胁迫下,两者的可溶性蛋白含量呈现上升趋势,表明植株在胁迫时通过合成大量的渗透物质以适应外界逆境环境;但转基因油菜植株的可溶性蛋白含量略高于非转基因植株。恢复供水后,干旱胁迫解除,植株各项生理功能逐渐恢复,可溶性蛋白含量逐渐下降(图5)。
3.5. 干旱胁迫对SOD和POD活性的影响
SOD是生物体内防御氧化损伤的主要酶类,它能使植物体内的自由基处于平衡状态。POD也是植物抗氧化系统的重要酶,它与植物的呼吸作用,光合作用及生长素的氧化等都有关系。自然干旱胁迫过程中,植株的SOD和POD活性都有所变化。随着胁迫时间的延长,油菜植株SOD和POD活性逐渐增大,胁迫到35 d时达到最大值,转基因和非转基因油菜植株的SOD分别达到279.95 U/mg∙protein和215.99 U/mg∙protein,POD的最大活性分别为208.70 U/mg∙protein和144.74 U/mg∙protein。此时,油菜植株受损较严重,叶沿卷曲,叶片萎焉较严重,叶片发黄,脱落。复水后,植株的干旱胁迫逐渐解除,未脱落的叶片随着复水时间的延长逐渐恢复,表现为叶片吸水膨胀,萎焉状况明显得到改善。此时,由于植株体内活性氧物质的减少,植株的SOD和POD活性也有所下降。但在整个胁迫过程中,转基因油菜的SOD和POD活性水平均高于非转基因油菜的活性。
4. 讨论
作物对非生物胁迫如干旱的耐受性在植株个体水平和细胞水平上都是很复杂的[1] [23] 。水分胁迫干扰了植物整体或部分生理生化过程,从而降低了植物的生长和产量[24] [25] 。干旱首先使土壤中的含水量逐渐下降,从而改变了植物体内的相对含水量,而植物组织相对含水量反映了植物内水分亏缺的程度。本研究中,在自然干旱条件下,土壤含水量的降低是一个缓慢渐进的过程,在0~14 d时期甘蓝型油菜的含水量降低不明显,但在14 d后,随着胁迫时间的延长,干旱程度不断加深,土壤含水量、油菜含水量均显著下降,但在整个过程中转基因油菜叶片的相对含水量比非转基因油菜的要高。到35 d时,植株叶
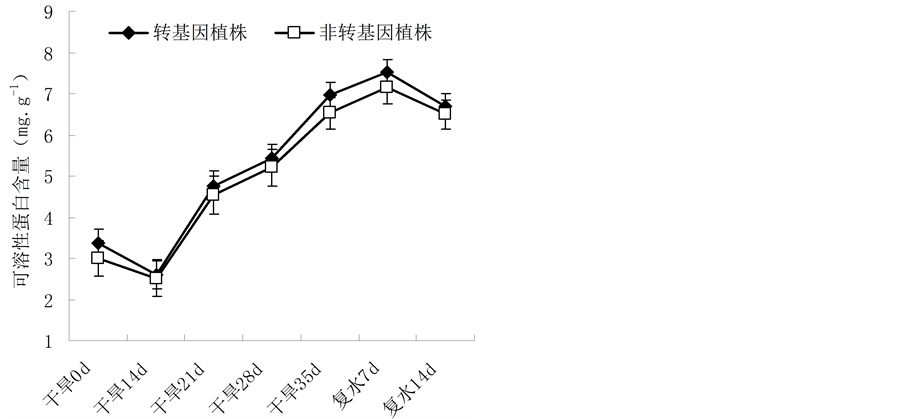
Figure 5. Change of the soluble protein content in leaves of Brassica napus L. during different periods of drought stress
图5. 不同胁迫时间内植株可溶性蛋白含量的变化
片呈萎焉状态,伴有叶片卷曲。恢复供水后,油菜植株也逐渐恢复生长活力并长出新叶,但转基因油菜要先于非转基因油菜恢复生长活力并长出新叶,这可能是IrrE基因在直接或间接地通过调节叶片相对含水量来增加油菜的耐旱性。干旱胁迫引起MDA含量升高,说明膜脂发生的过氧化作用增强,对膜和细胞造成进一步的伤害,严重时使植物的细胞膜结构遭到破坏[15] 。非转基因油菜植株中MDA的含量比转基因植株中的MDA含量高,这表明非转基因油菜植株比转基因油菜植株的质膜受损严重,这与超表达AVP1基因提高转基因百脉根的耐盐性和抗旱性的结果相一致[26] 。
相应地,随着土壤和植物叶片含水量的变化,植物体内的叶绿素含量、可溶性蛋白含量以及SOD和POD活性也发生改变。在0~35 d的胁迫过程中,他们的含量和活性都随着土壤含水量的下降而逐渐升高,表明土壤和油菜叶片含水量的降低引起了干旱胁迫。干旱、盐渍等非生物胁迫和病虫害等生物胁迫都会影响细胞内蛋白质代谢的变化[25] ,自然干旱胁迫也引起了可溶性蛋白含量的增加,而可溶性蛋白含量是植物体代谢过程中蛋白质损伤的重要指标,其变化可以反映细胞内蛋白质合成、变性及降解等多方面的信息[27] 。同时,在本研究中,自然干旱胁迫也使叶绿素含量随着含水量的降低而减少,从而可能降低了植物的光合作用速率,与前人的研究结果一致[28] [29] 。而水分亏缺是引起植物的光合作用速率降低的主要原因,其主要表现在于植物在受到水分亏缺时气孔关闭[30] -[33] 、1,5-二磷酸核酮糖羧化酶/加氧酶的酶活性受到抑制[34] 以及ATP的合成出现障碍[35] 。植物遭受干旱等逆境胁迫时,植物光合作用和呼吸作用所产生的过多电子会在传递过程中产生大量的活性氧[36] [37] 。过量的活性氧对植物产生严重的氧化伤害,为了减轻或免于受到氧化伤害,植物启动了自身的防御系统:活性氧清除酶系统和非酶系统。其中SOD和POD是清除活性氧酶系统中最重要的酶类。本研究结果显示,在自然干旱胁迫下,油菜幼苗叶片中SOD和POD抗氧化酶的活性与植物抗氧化胁迫能力相关联,它们的活性高低可作为衡量植物抗逆性强弱的重要指标。由图6中的结果可知,油菜幼苗在受到干旱胁迫时,其SOD和POD活性都增加了,随着胁迫时间的延长和胁迫程度的加重,抗氧化酶活性逐渐增加,二者的酶活性35 d后时达到最高。但在整个过程中转基因油菜叶片的叶绿素含量、可溶性蛋白含量以及SOD、POD活性均比非转基因油菜的高,说明在干旱胁迫下,IrrE基因是通过增加油菜叶片的叶绿素含量、可溶性蛋白含量以及SOD、POD活性来提高转基因油菜的抗旱能力。

Figure 6. Changes of SOD and POD activity in leaves of Brassica napus L. during different periods of drought stress
图6. 不同干旱胁迫时间内油菜植株SOD和POD活性变化
基金项目
本研究基金项目有:973项目(2013CB733903),863项目(2012AA063503),国家转基因专项(2014ZX0801201B),农业部公益性行业科研专项(201103007)和西南科技大学博士研究基金(11zx7104)和四川省生物质资源利用与改性工程技术研究中心开展开放基金(编号:13zxsk04)。