1. 引言
质子泵抑制剂( proton pump inhibitors, PPIs),是二十多年来临床应用广泛、疗效较好的药物,主要用于治疗酸相关性疾病。PPIs与以往临床应用的抑制胃酸药物如组胺H2受体拮抗剂相比,其抑酸作用强、起效快,治愈率高,这类药物可高效、快速地抑制胃酸分泌和清除幽门螺杆菌,广泛用于胃溃疡、十二指肠溃疡、反流性食管炎和卓-艾综合征等疾病的治疗[1] [2] 。目前,已上市的PPIs主要有奥美拉唑、兰索拉唑、泮托拉唑、雷贝拉唑和埃索美拉唑。各种PPIs的结构类似,都是在苯并咪唑环上进行结构衍生,即在苯环和吡啶环进行不同基团修饰。
S-泮托拉唑(S-pantoprazole, Scheme 1)化学名为(S)-5-二氟甲氧基-2-[[(3,4-二甲氧基-2-吡啶基)-甲基]亚磺酰基]-1H-苯并咪唑,是一种抑制胃酸分泌的质子泵抑制剂,临床主要用于治疗胃溃疡和十二指肠溃疡等消化性系统疾病。研究表明,(S)-构型的泮托拉唑同消旋体泮托拉唑以及(R)-构型的泮托拉唑相比较,其疗效更好,抑酸作用强,生物利用度高,毒副作用低[3] -[7] 。
目前报道的制备S-泮托拉唑主要有两种方法:(1) 对消旋体进行手性拆分;(2) 对前手性硫醚进行不对称氧化,使用这两种方法都可以获得单一对映体或富含单一对映体的产物。本文对S-泮托拉唑的制备研究现状进行了概述。
2. 拆分法制备S-泮托拉唑
1992年,Kohl B.等人[8] 报道了消旋体泮托拉唑钠盐与天然手性源(+)-葑醇的衍生物——葑基氯甲醚反应,合成出甲氧基位于5或6位的(+)-二氟甲氧基-2-[[(3,4-二甲氧基-2-吡啶基)-甲基]亚磺酰基]-1-[(+)甲氧基-葑基]-苯并咪唑,将所得到的非对映异构体经硅胶柱层析提纯、分步结晶后,再经水解得到(S)-泮托拉唑,[α]D22 = −144.4˚ (C = 0.5,乙腈/甲醇 = 1:1),见(Scheme 2)。
2008年,山东新时代药业有限公司[9] 公开了一种通过拆分消旋体的方法获得S-泮托拉唑的技术(Scheme 3)。他们采用手性拆分试剂S-1,1,2-三苯基-1,2-乙二醇,与消旋泮托拉唑在合适的溶剂中反应,过滤出生成的S-泮托拉唑。S-1,1,2-三苯基-1,2-乙二醇复合物沉淀,该复合物沉淀经过酸碱作用,得到中性的S-泮托拉唑,该方法拆分收率高达38%,产品单一异构体比例高达99%或以上,工艺简单。
2008年,陕西新安医药科技有限公司[10] 以联二萘酚化合物为包结主体,以外消旋的泮托拉唑为客体,采用包结拆分的方法制得对映选择性高达98.8%的S-泮托拉唑(Scheme 4)。
2011年,专利WO2011042910A2 [11] 描述了一种用手性拆分剂R-BNPPA拆分消旋泮托拉唑获得S-泮托拉唑的方法(Scheme 5),在苯和环己烷按一定比例混合的溶剂中,消旋的泮托拉唑与手性R-BNPPA

Scheme 1. S-泮托拉唑
Scheme 1. S-Pantoprazole

Scheme 2. Resolution of pantoprazole with fenchyl alcohol derivatives
Scheme 2. 葑醇衍生物拆分泮托拉唑

Scheme 3. Resolution of pantoprazole with S-1, 1, 2-Triphenyl-1, 2-ethanediol
Scheme 3. S-1,1,2-三苯基-1,2-乙二醇拆分泮托拉唑

Scheme 4. Resolution of pantoprazole with BINOL
Scheme 4. 联二萘酚拆分泮托拉唑

Scheme 5. Resolution of pantoprazole with chrial R-BNPPA
Scheme 5. 手性R-BNPPA拆分消旋泮托拉唑
反应生成非对映异构体复合物,其手性纯度高达98%。对此复合物进行部分重结晶,从而获得S-泮托拉唑。R-BNPPA的复合物。然后将分离出来的异构体在乙酸乙酯和水的混合溶剂中用碱解离得到S-泮托拉唑。
3. 不对称氧化
S-泮托拉唑属于手性亚砜类化合物,其手性中心是亚砜基团的硫原子。目前报道的构建手性亚砜的方法主要有:(1) 手性亚磺酰衍生物的亲核取代[12] ;(2) 硫醚的不对称氧化,包括酶催化氧化[13] 、硫醚的非对映选择性氧化[14] 、手性氧化剂对映选择性氧化[15] 、手性金属络合物催化的潜手性硫醚不对称氧化[16] 。以上所述的手性亚砜的制备方法,除了酶催化氧化硫醚和金属催化剂催化的硫醚不对称氧化之外,其他方法需要化学计量的手性助剂。迄今为止,制备具有光学活性的亚砜,最有效、最经济的方法是对映选择性的催化氧化硫醚,而各种酶和各类手性金属催化剂因其具有高效的催化作用,一直得到人们的关注。通过对泮托拉唑前体硫醚的不对称催化氧化获得单一构型的泮托拉唑是效率最高、最经济的方法。
1996年,Larsson等[17] 以Kagan体系(Ti(O-iPr)4/(S,S)-DET/H2O = 1/2/1(摩尔比),在一个当量的金属钛手性络合物存在下,用叔丁基过氧化氢作氧化剂[18] [19] 为基础,在反应体系中加入适量的碱,大大提高了反应效果。将该体系用于制备S-泮托拉唑时(Scheme 6),得到化学含量为89%,光学纯度为86%的亚砜粗产物,经过一定的后处理和重结晶步骤后对映体过量能够达到97.4%。
2003年,Kohl, B. et al. [18] 报道了一种利用手性锆络合物或手性铪络合物制备S-泮托拉唑的新方法,获得了光学纯度大于90%的S-泮托拉唑(Scheme 7)。他们用(+)-L-酒石酸衍生物作为手性配体,异丙苯过

Scheme 6. Preparation of S-pantoprazole with chiral Titanium complexes
Scheme 6. 手性钛络合物制备S-泮托拉唑
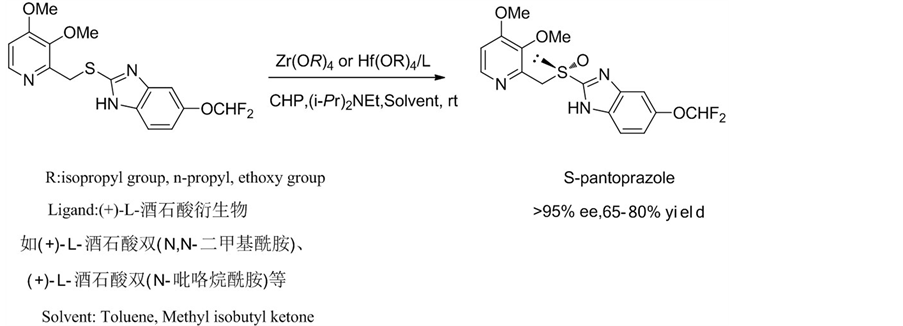
Scheme 7. Preparation of S-pantoprazole with chiral Zirconium or Hafnium complexes
Scheme 7. 手性锆络合物或手性铪络合物制备S-泮托拉唑
氧化氢作为氧化剂,使用金属锆络合物,在室温下进行反应,加入三乙胺或者N,N’-二异丙基乙胺,得到对映选择性大于95%的S-泮托拉唑。
2007年,沈阳药科大学[19] 在专利CN 200710010273.4中报道了一种在手性氨基醇和烷氧基钛或烷氧基锆化合物存在下催化氧化相应前手性硫醚化合物得到单一对映体或对映体富集形式的手性亚砜类质子泵抑制剂,包括S-泮托拉唑的的合成方法(Scheme 8)。此方法用到了一种新颖的手性氨基醇配体(如S-2-氨基丙醇、S-2-氨基-1-丁醇、S-苯甘氨醇等),可得到光学纯度大于90%的S-泮托拉唑,经过结晶纯化可使光学纯度达到97%以上。
2008年,Emcure House [20] 报道了一种用手性氮氧丙烷作为氧化剂,在合适的溶剂和碱的条件下,选择性的氧化硫醚制备手性亚砜的方法。该方法指出,针对具有不同官能团的前手性硫醚需用不同溶剂条件,需使用特定的碱以及特定的氧化剂。在异丙醇中,用特定碱1,8-二氮双环[5.4.0]-7-十一烯(DBU),氧化剂为((R)-(-)樟脑磺酰基)氮氧环丙烷,氧化泮托拉唑硫醚,经过一定的纯化后处理,可以得到98.62%的对映体过量的S-泮托拉唑(Scheme 9)。
2008年,中科院上海有机所姜标等[21] ,通过对Rosini用于苯基甲基硫醚不对称催化氧化的(1R,2R)-1,2-二苯基乙二醇-钛体系进行改进,实现了对S-泮托拉唑等其他质子泵抑制剂的不对称氧化合成(Scheme 10)。该小组首先对各种苯环邻位取代的(1R,2R)-1,2-二苯基乙二醇配体进行了系统的筛选,发现当该手性二醇配体的苯环上2-位被溴原子取代时效果最好。再对溶剂、温度、时间、氧化剂等进行优化。在−20℃,0.5%的催化剂存在下,不加入有机碱,用2当量的70%的TBHP作氧化剂,在甲苯溶剂中将

Scheme 8. Preparation of S-pantoprazole with chiral amino alchols
Scheme 8. 手性氨基醇制备S-泮托拉唑

Scheme 9. Preparation of S-pantoprazole with chiral nitrogen oxygen propane
Scheme 9. 手性氮氧丙烷制备S-泮托拉唑

Scheme 10. Preparation of S-pantoprazole with (1R,2R)-1,2-two phenyl ethylene glycol-Titanium system
Scheme 10. (1R,2R)-1,2-二苯基乙二醇-钛体系制备S-泮托拉唑
泮托拉唑硫醚前体氧化24小时,对映体过量可达92%,产率90%。
邓金根等[22] -[24] 发展了一种用于不对称催化氧化制备手性亚砜类产品的有效方法。2009年,其报道了以四异丙基钛酸酯和酒石酸正丙酰胺形成的手性络合物,用枯烯酸过氧化氢作氧化剂氧化前手性泮托拉唑硫醚,其对映选择性只有70%。之后邓金根小组先后报道了以一系列酒石酸酰胺为配体,不对称氧化泮托拉唑硫醚,可得到对映选择性达到96.5%以上的(S)-泮托拉唑(Scheme 11)。
2014年,卢进城[25] 报道了对(S)-泮托拉唑钠的不对称氧化合成工艺的改进(Scheme 12),用(1S,2S)-(+)-2-氨基环己酸盐酸盐与Mo-HTC钼离子与对甲苯磺酸根预柱撑的镁铝类水滑石进行离子交换

Scheme 11. Preparation of S-pantoprazole with chiral Titanium complexes
Scheme 11. 手性钛络合物制备S-泮托拉唑

Scheme 12. Preparation of S-pantoprazole sodium with catalytic asymmetric oxidation
Scheme 12. (S)-泮托拉唑钠的不对称氧化合成
而得到催化体系,替代了酒石酸/异丙酸钛,使催化活性更高,用次氯酸钠氧化制备泮托拉唑,进而将其制备成S-泮托拉唑钠盐,其对映选择性高达99.7%。
4. 结语
(S)-泮托拉唑的制备经过二十多年的探索与研究,已经取得了很大的发展。通过手性拆分的方法获得光学纯的泮托拉唑是一种比较有效、快速的方法。但此方法具有很多不可避免的缺点:操作复杂、产率不高、理论产率最高只能达到50%,分离步骤复杂繁琐,分离出来的相反异构体回收循环使用困难而形成浪费。
随着不对称催化技术的发展,多种手性金属络合物催化剂被应用于(S)-泮托拉唑的制备,包括手性钛络合物、手性锆络合物和手性铪络合物等。相对而言,手性钛络合物的研究较为深入。
总之,目前对于S-泮托拉唑的制备技术,特别是不对称催化氧化制备技术已经取得了突破性成果,实现了大规模制备。但目前使用的手性金属络合物和手性配体仍有一些不足之处,如环境污染,可重复使用率低,价格昂贵等。因此,寻找高效、绿色、环保且经济实用的催化体系进行泮托拉唑硫醚的不对
基金项目
常州市应用基础研究计划项目。