1. 引言
长期以来,社会发展中的不科学手段使得人类生存环境不断恶化,发展环境修复技术才能确保社会健康发展并可持续延伸。半导体光催化利用丰富的自然光或室内人工照明中合适的带隙能量来激发出电子–空穴对,其迁移到半导体表面后会引发或加速特定氧化还原反应,有效分解或氧化有毒物质从而修复环境。这种光催化已成为多种绿色地球和再生能源项目中最有前途的技术之一。太阳光入射到地球的能量中,紫外线、可见光和近红外线的比例分别为:5%、48%与44% [1] [2] 。因此,选则一种能够充分利用可见光的材料显得十分有意义。铁元素在地壳中含量非常丰富,来源丰富、价格低廉、对环境无毒害,其氧化物α-Fe2O3结构稳定,带隙宽度约为2.2 eV,能够很好地响应可见光。目前,不同形貌的α-Fe2O3纳米材料已经采用固相法、水热溶剂热法、水解法、共沉淀法等制备了 [3] - [8] 。例如,Xu等 [9] 采用水热法,以C4MimBF4为添加剂,合成了α-Fe2O3空心多面体,在可见光下,对RhB具有很好的降解效果。Yang等 [10] 以水热法制备的FeCO3为前驱体,在650℃热分解得到了具有非传统形貌的α-Fe2O3微米球。亦可先合成前驱体α-FeOOH或β-FeOOH [11] [12] ,进而通过热处理可得到α-Fe2O3。
这些合成方法相比较来说,固相合成工艺简单、产率高,能够容易的得到粒径较小的纳米材料 [13] - [17] 。因此,本文我们试图通过固相反应首先得到前驱体,进而通过热分解获得α-Fe2O3纳米材料,研究不同温度热处理对材料结晶、光学和可见光光催化降解亚甲基蓝的影响。
2. 实验
2.1. α-Fe2O3的合成
分别称取1.988 g FeCl2∙4H2O (10 mmol)和1.423 g Na2S2O8 (6 mmol),置于玛瑙研钵中研磨约5 min,然后依次加入加入1.442 g SDS (5 mmol)和1 ml的去离子水,继续研磨30 min,随着研磨的进行,混合物的颜色逐渐变成黄色,证明了固相反应的发生。将产物用去离子水洗涤、过滤,室温干燥即可得到前驱体。然后将前驱体置于马弗炉中450℃~600℃加热2 h即可得到产物α-Fe2O3。
2.2. 样品表征
本实验采用日本理学Miniflex 600型X-射线衍射仪对所制备的样品进行物相表征。测试条件:Cu靶Kα激发,电压、电流分别为40 kV、15 mA,衍射角2θ的范围为10˚~80˚,扫描速度为5˚/min。采用德国Bruker VERTEX 70型红外光谱仪鉴别样品表面与分子中的官能团或化学键,波数范围:400~4000 cm−1。采用Hitachi S-4800型场发射扫描电子显微镜对样品进行微观形貌表征。采用美国Perkin-Elmer公司的Lambda 950 UV-Vis-NIR分光光度计,对样品进行光学性能测试,参比物为BaSO4,波长范围:250~800 nm。
2.3. 光催化测试
光催化实验在自行搭建的光催化实验装置上进行,采用150 W氙灯在300 ml的夹层烧杯中进行光催化反应,并用滤波片滤掉λ < 420 nm的光,本实验采用的亚甲基蓝溶液浓度为7 mg/L、体积为120 ml,光催化剂用量为20 mg。光催化实验开始前,将制备的α-Fe2O3光催化剂置于装有亚甲基蓝的夹层烧杯中,黑暗条件下磁力搅拌1 h,使得α-Fe2O3粉末与亚甲基蓝分子间的吸附–解吸过程达到动态平衡。然后从中取出4 ml上层溶液置于离心管内,以9000 r/min的转速,离心5 min,取上清液用UV-vis 2550型紫外–可见分光光度计进行吸光度测试。光催化实验开始后,每隔45 min取4 ml待测模拟污染物,离心后取上清液测试吸光度,记录波长663 nm处的吸光度值A。根据拟合得到的吸光度A与浓度C的方程表达式:
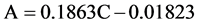
判定系数R2 = 0.99871,相关系数R = 0.99935
之后再根据降解率公式:
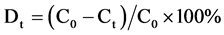
就可以计算出光催化降解率。其中,C0是光催化剂与亚甲基蓝分子达到吸附–解吸平衡后的浓度,Ct是光催化进行到t时刻时的浓度。
3. 结果与讨论
3.1. X-射线衍射分析(XRD)
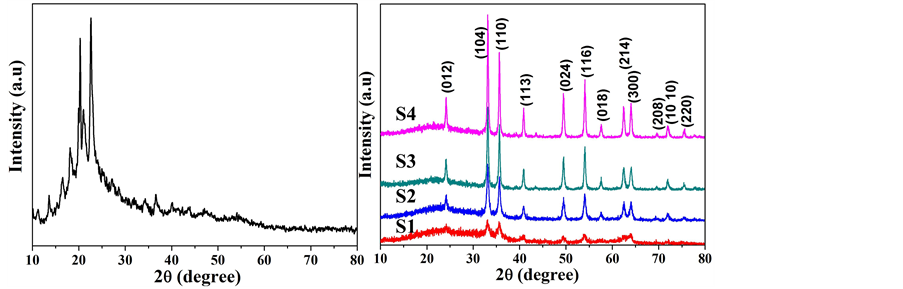
图1(a)是前驱体XRD图谱,我们发现,没有反应物的衍射峰出现,说明通过研磨过程的确发生了反应,得到了新的物质。对其加热后得到的XRD图谱如图1(b)所示,与标准PDF卡片(NO. 33-0664)对照发现,在24.138˚、33.152˚、35.611˚、49.479˚、54.089˚、62.449˚和63.989˚处的衍射峰分别对应于六方相α-Fe2O3的(012)、(104)、(110)、(024)、(116)、(214)和(300)晶面。从图中还可以看出,随着热处理温度的升高,衍射峰的强度逐渐增强,并且(104)晶面的衍射峰强度总是比(110)晶面的衍射峰强度高,说明产物
 (a) (b)
(a) (b)
Figure 1. (a) XRD pattern of the precursor; (b) XRD patterns of the as-prepared products S1-S4 obtained at 450˚C - 600˚C
图1. (a) 前驱体的XRD图谱;(b) 450℃~600℃所得产物S1-S4的XRD图谱
的结晶性在逐渐增强,并且具有(104)晶面择优取向。根据Scherrer公式,从(104)和(110)晶面计算了产物的晶粒大小。结果如表1所示,产物α-Fe2O3的晶粒尺寸处于纳米级,随热处理温度升高,尺寸在逐渐增大。
3.2. 傅里叶转换红外线光谱分析(FTIR)
图2为前驱体的FTIR光谱图,可以鉴别样品表面的官能团。从图中可以看出,样品在3251和1650 cm−1处的吸收峰对应于结晶水中O-H键的弯曲和伸缩振动峰。2848、2921和1469 cm−1处的吸收峰对应的是残留表面活性剂SDS中的-CH2-和-CH3的伸缩振动峰。1071和1220 cm−1的吸收峰对应于-OSO3的振动峰,587 cm−1处的吸收峰对应Fe-O的伸缩振动峰,870和980 cm−1的吸收峰可能是由Fe原子与O-H键的弯曲振动引起的。
3.3. α-Fe2O3的微观形貌表征与分析
图3是所得α-Fe2O3纳米粒子的FESEM图,前驱体在450℃下热处理得到的α-Fe2O3纳米粒子轮廓不是很明显(如图3(a))。当热处理温度升到500℃时,如图3(b),α-Fe2O3纳米粒子的轮廓已经能够明显看出。当继续升高温度到550℃时,α-Fe2O3纳米粒子清晰可见,球型颗粒直径约为28 nm (如图3(c))。当600℃时,可以看出(图3(d)),α-Fe2O3纳米粒子的球型团聚颗粒,粒子直径约为36 nm,这与表1中的结果比较符合。
3.4. 紫外–可见漫反射吸收光谱分析(DRS)
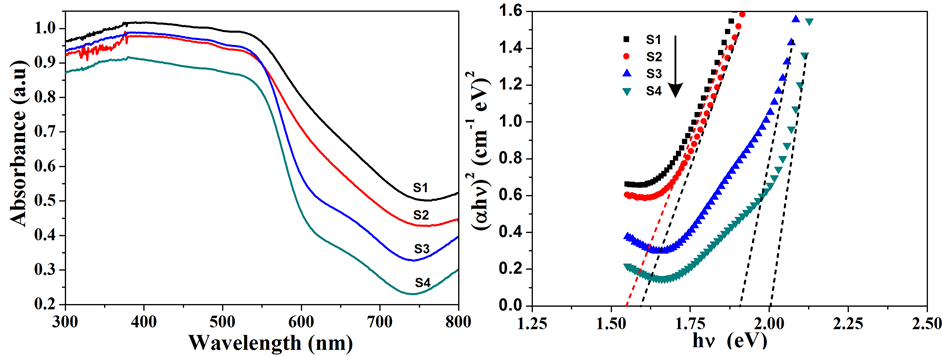
紫外–可见漫反射吸收光谱能够直观的反映出样品对紫外可见光的吸收情况。如图4(a)所示,α-Fe2O3纳米粒子在300~550 nm范围内有强烈的吸收,波长大于550 nm后吸收有所减弱,并且随着加热温度的升高,吸收强度逐渐变弱,这可能来源于它们带隙的变化。根据Tauc方程,构建(αhν)2-hν曲线可以从中得到不同α-Fe2O3纳米粒子的带隙宽度。如图4(b)所示,可得产物S1,S2,S3和S4的带隙宽度分别为1.55、1.60、1.91 和2.01 eV,均小于体相α-Fe2O3的带隙宽度(2.2 eV)。并且,随着温度的升高,S1~S4的带隙宽度逐渐增大,如图4(c)所示,因而导致了对大波长可见光的吸收能力变弱。热处理温度影响着产物的结晶度与粒子大小,因而调制着产物的带隙宽度。
3.5. α-Fe2O3纳米粒子的光催化性能研究
α-Fe2O3纳米粒子对亚甲基蓝的可见光光催化降解曲线和降解率如图5(a),图5(b)所示。可见光照射

Table 1. The average crystallite size of the α-Fe2O3
表1. α-Fe2O3的平均晶粒尺寸
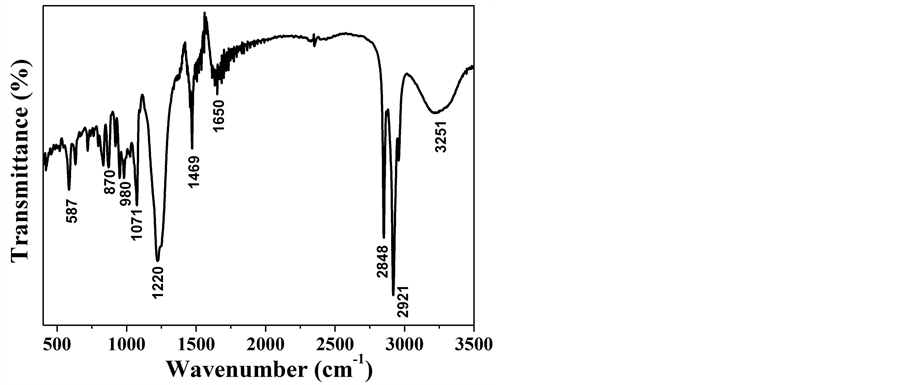
Figure 2. FTIR spectra of the precursor
图2. 前驱体的傅里叶转换红外线光谱

Figure 3. FESEM images of the α-Fe2O3 nanoparticles (a) S1; (b) S2; (c) S3; (d) S4
图3. α-Fe2O3纳米粒子的FESEM图片 (a) S1;(b) S2;(c) S3;(d) S4
 (a) (b)
(a) (b)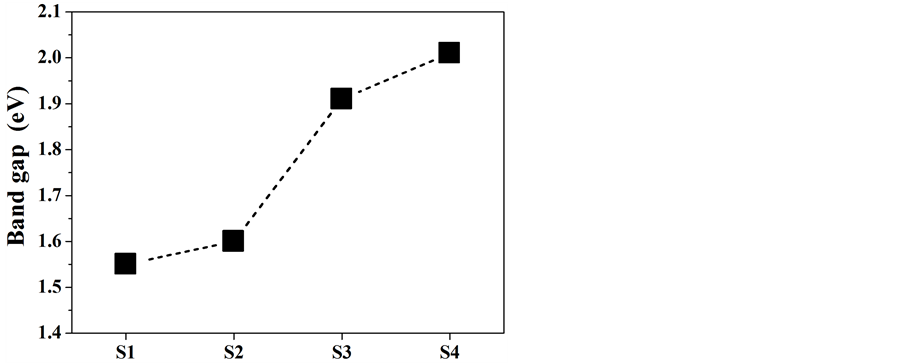 (c)
(c)
Figure 4. Optical properties and band gap spectra of the α-Fe2O3 nanoparticles (a) UV-vis diffuse reflection spectra of the products S1-S4; (b) Curves of (αhν)2 vs. hν of the products S1-S4; (c) Band gap tendency curve of the α-Fe2O3 nanoparticles
图4. α-Fe2O3纳米粒子的光学性能及带隙图谱 (a) 产物S1-S4的DRS图谱;(b) 产物S1-S4的(αhν)2 vs. hν曲线;(c) α-Fe2O3纳米粒子的带隙走势图
 (a) (b)
(a) (b) (c)(d)
(c)(d)
Figure 5. Visible-light photocatalytic properties images of the α-Fe2O3 nanoparticles (a) Curves of relative photodegradation rate of MB; (b) Histograms of photodegradation rate of MB; (c) First-order kinetics fitting curves of photodegradation of MB; (d) Tendency curves of the degradation rate constants k, inset is the schematic diagram of photo-excited electron-hole separation and recombination in the photodegradation of MB dyes process
图5. α-Fe2O3纳米粒子的可见光光催化性能图 (a) 亚甲基蓝相对降解率曲线;(b) 亚甲基蓝降解率直方图;(c) 降解亚甲基蓝一级动力学拟合图;(d) 反应速率常数k的变化趋势图,插图为光降解亚甲基蓝过程的光激发电子–空穴分离、复合示意图
条件下亚甲基蓝的自降解率为14.8%,加入α-Fe2O3纳米粒子光照180 min后,对亚甲基蓝的降解率明显提高,样品S1的光催化效率为57.23%,而样品S2具有最高的可见光光催化效率70.60%,温度升高后得到的样品S3和S4光催化效率有所降低,分别为68.72%和67.43%。一般认为,样品的光催化效率与其能带结构有关。在可见光照射下,样品会产生光生电子–空穴对,一部分电子–空穴对立即复合,一部分迁移到催化剂表面,直接氧化分解MB,或产生超氧自由基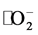 分解MB,只有当半导体材料具有合适的带隙宽度时,电子–空穴复合率才会最低,从而使其具有最高的光催化效率,如图5(d)插图所示。
分解MB,只有当半导体材料具有合适的带隙宽度时,电子–空穴复合率才会最低,从而使其具有最高的光催化效率,如图5(d)插图所示。
光催化降解亚甲基蓝符合一级动力学方程:

其中,C0和C分别为模拟污染物亚甲基蓝溶液的初始浓度和t时刻的浓度,t为光催化降解时间,k为光催化降解过程的反应速率常数。通过计算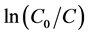 并对时间t做线性拟合,可以得出不同产物光催化降解亚甲基蓝的一级动力学曲线和整个过程的反应速率常数k。从图5(c)中可以看出,样品S1,S2,S3和S4的反应速率常数k分别为4.69 × 10−3、6.78 × 10−3、6.48 × 10−3和6.20 × 10−3 min−1。其变化趋势如图5(d)所示,在500℃下热处理所得产物S2的k值最大,与降解效率的变化趋势类似。
并对时间t做线性拟合,可以得出不同产物光催化降解亚甲基蓝的一级动力学曲线和整个过程的反应速率常数k。从图5(c)中可以看出,样品S1,S2,S3和S4的反应速率常数k分别为4.69 × 10−3、6.78 × 10−3、6.48 × 10−3和6.20 × 10−3 min−1。其变化趋势如图5(d)所示,在500℃下热处理所得产物S2的k值最大,与降解效率的变化趋势类似。
4. 结论
1) 通过前驱体的热分解获得了不同尺寸的α-Fe2O3纳米粒子,热处理温度升高,粒子晶粒尺寸逐渐增大。
2) 热处理温度升高,α-Fe2O3纳米材料的带隙宽度逐渐升高,但均小于体相α-Fe2O3的带隙宽度(2.2 eV)。
3) 500℃热处理2 h所得α-Fe2O3纳米材料具有最高的可见光光催化效率和光降解速率常数,其分别为70.60%和6.78 × 10−3 min−1。
4) 太高或太低的热处理温度均会导致光催化效率降低,这主要受带隙大小和电子–空穴复合机率影响。
致谢
感谢江苏省博士后创新基金(1202016C)、江苏高校优势学科建设工程资助项目对本文的资助。
*通讯作者。