1. 引言
α,β-不饱和醛选择性加氢生成不饱和醇是精细化学品制备的关键反应之一 [1] 。典型的α,β-不饱和醛如肉桂醛(CAL),其选择性加氢产物肉桂醇(COL)广泛应用于香精、香料、化妆品、医药、杀菌剂的合成,是一种重要的化工中间体。肉桂醛加氢反应路径如图1所示。从热力学的角度来看,肉桂醛结构同时含有C=C和C=0双键,二者共轭,前者键能(615 KJ/mol)比后者键能(715 KJ/mol)小,更易发生加氢反应。肉桂醛加氢有多种可能产物,如氢化肉桂酵(HCAL)、肉桂醇(COL)和氢化肉桂醇(HCOL)等,探索新型加氢催化剂的设计与制备,改善加氢反应的活性与选择性,对理论研究和工业应用均有十分重要的意义。
石墨相氮化碳(g-C3N4)是一种聚合物半导体材料,比表面积较大,除骨架氮外,表面含-NH2、-NH和吡啶等官能团 [2] 。作为催化剂载体,g-C3N4具有与氮原子掺杂的其他碳质载体相似的优点,如利于贵金属分散等。引入的N原子,导致氮化碳材料表面具有碱性位点,而这些位点在催化应用中既可以作为潜在的活性位点,也可以和活性金属结合,增强金属在材料表面的附着,促进金属位点的活性;另外,氮的引入可以改变材料的能带结构使材料的价带降低,增强材料的化学稳定性及增加费米能级上的电子密度 [3] 。g-C3N4主要应用于可见光催化 [4] 、电化学 [5] 等领域,作为新型的加氢催化剂载体也渐受关注。Wang等使用介孔聚合g-C3N4(mpg-C3N4)负载Pd,并用于催化苯酚选择性加氢制备环己酮,选择性和转
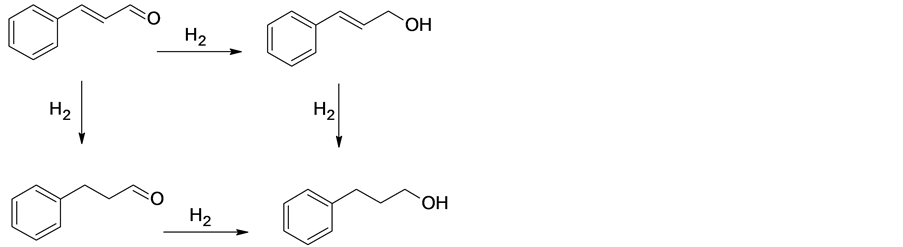
Figure 1. Reaction pathways in the hydrogenation of CAL
图1. 肉桂醛加氢反应路径图
化率都达到99%以上 [6] ;Deng等研究了Pd@mpg-C3N4催化苯乙炔半加氢到苯乙烯的反应,苯乙炔转化率 > 99%,苯乙烯选择性为94% [7] 。g-C3N4负载的其他金属用于催化加氢反应,目前仍较少报道。本文以尿素为前驱体,高温煅烧制备石墨相的g-C3N4,实验过程如图2所示。再采用乙二醇还原法载铂,制备Pt/g-C3N4材料(过程如图3所示)。通过XRD、TEM、FT-IR、XPS和ICP-AES等手段,对Pt/g-C3N4的组成和结构进行了分析,并以肉桂醛加氢为探针反应,研究了制备条件如g-C3N4煅烧温度对催化性能的影响以及催化剂的重复使用性能,探索Pt/g-C3N4作为选择性加氢催化剂的可能性。
2. 实验部分
2.1. 试剂与仪器
尿素、乙醇和乙二醇(EG),国药集团化学试剂有限公司;氯铂酸(H2PtCl6)和肉桂醛,阿拉丁试剂公司。所有试剂均为分析纯,使用前未经处理。
中国飞利浦公司Philips PW3040/60型粉末衍射仪;日本日立高科公司HITACHI S-4800型扫描电子显微镜;日本电子JEM-2100F型透射电子显微镜;美国尼高力公司NEXUS型智能傅里叶变换光谱仪;美国的IRIS Intrepid ⅡXSP型号电感耦合等离子体原子发射光谱;中国赛默飞世尔科技公司ESCLALAB 250Xi型X射线光谱仪和中国Autosorb-I-MP型自动吸附比表面和孔隙度分析仪。
2.2. 催化剂Pt/g-C3N4催化剂的制备
取一定量的尿素置于坩埚中,半封闭状态下在马弗炉中煅烧。以15℃/min的速率升温至设定温度(450℃~600℃),保持两个小时,最后冷却至室温得到黄色粉末状产品。根据煅烧温度,所制样品分别标记为g-C3N4-450℃、g-C3N4-500℃、g-C3N4-550℃和g-C3N4-600℃。
适量H2PtCl6溶液和EG共置于烧杯中搅拌待用;将一定量的g-C3N4和EG置于圆底烧瓶中,超声、机械搅拌至混合均匀,冷凝回流下逐步升温,并逐滴加入H2PtCl6-EG混合溶液,160℃冷凝回流3 h后,冷却至室温,分离所得固体物用去离子水和乙醇分别洗涤若干次,70℃真空干燥12 h,得到催化剂。根据ICP-AES检测实际含铂的质量分数,根据实际含量分别标记为4.3 wt.% g-C3N4-450℃、4.2 wt.% g-C3N4- 500℃、4.2 wt.% g-C3N4-550℃和4.3 wt.% g-C3N4-600℃。
2.3. 催化加氢反应
取0.1 g 4.2 wt.% Pt/g-C3N4催化剂、2 mL水、16 mL乙醇和2 mL肉桂醛置于50 mL高压反应釜中。用0.6 MPa氢气置换釜内空气若干次后,使反应釜升温至80℃并保持恒定。开启搅拌,充入1.6 MPa氢气,开始反应计时。反应完毕后,关闭加热电源,放掉高压釜内剩余氢气。当釜温降到室温后打开反应
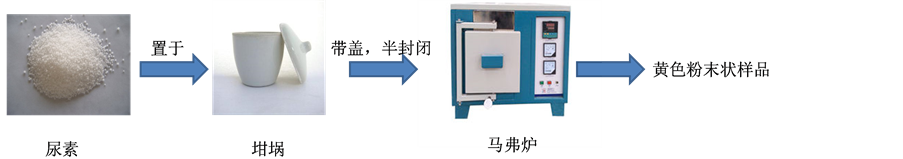
Figure 2. Schematic illustration of the synthetic process of g-C3N4 carrier
图2. 载体g-C3N4制备过程示意图
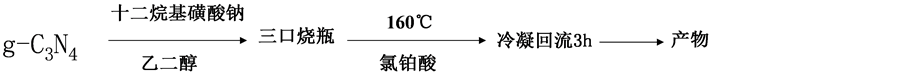
Figure 3. Schematic illustration of the synthetic process of Pt/g-C3N4 catalysts
图3. 催化剂Pt/g-C3N4制备过程示意图
釜用滴管取样,反应混和液在带有FID检测器的岛津GC-2014型气相色谱仪上分析,进样量为0.4 μl,以联苯为内标物进行定量分析。
1) 转化率的计算方法

Y:反应中肉桂醛的转化率
m1:反应物中投入的肉桂醛量(g)
m2:反应后剩余的肉桂醛量(g) (由气相色谱定量分析得)
2) 产物选择性的处理方法:
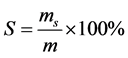
S:产物的选择性
ms:由反应后得到产物实际量(g) (由气相色谱定量分析得)换算成相应地肉桂醛量
m:各生成物所消耗的总的肉桂醛的量(g)
3. 结果与讨论
3.1. 催化剂表征
图4为450℃~600℃下煅烧所得g-C3N4,以及550℃下煅烧的g-C3N4负载4.2% Pt (4.2 wt.% Pt/g-C3N4- 550℃)催化剂在使用前后的XRD图。由图可知,27.6˚处为g-C3N4 (002)特征衍射峰,对应层间距为0.33 nm;13.0˚处为g-C3N4 (100)特征衍射峰,对应于三-s-三嗪单元间的大环结构 [5] 。在450℃~600℃间,不同煅烧温度下均可以形成g-C3N4。负载活性金属Pt后,g-C3N4特征衍射峰无明显变化,表明g-C3N4的物理结构不变。在39.7˚处有明显的衍射峰,归属为Pt(111);46.1˚处的衍射峰归属为Pt(200);另外在67.4˚和81.9˚处的衍射峰分别归属为Pt(220)和Pt(311) [8] 。通过拟合知,新鲜催化剂与使用3次的催化剂,Pt(111)晶面的半峰宽相差0.02弧度,变化微小,表明晶体粒径基本保持不变。
图5(a)、图5(b)分别为g-C3N4-550℃的SEM和TEM图。由图可知,g-C3N4形貌呈与树叶相似的片状,且边缘较厚。图5(c)、图5(d)为催化剂4.2 wt.% Pt/g-C3N4-550℃的TEM图,可明显观察到金属铂颗粒均匀分散在g-C3N4表面,无团聚现象,粒径约为2~3 nm,高分辨透射电镜图像证实了形成的金属铂纳

Figure 4. XRD patterns of the g-C3N4-450˚C, g-C3N4-500˚C, g-C3N4-550˚C and g-C3N4-600˚C ((a)-(d)), fresh and used 4.2 wt.% Pt/g-C3N4 catalysts ((e), (f))
图4. (a)~(d)分别是g-C3N4-450℃、g-C3N4-500℃、g-C3N4-550℃和g-C3N4-600℃的XRD,(e)、(f)分别为新鲜、后催化剂4.2 wt.% Pt/g-C3N4-550℃的XRD图
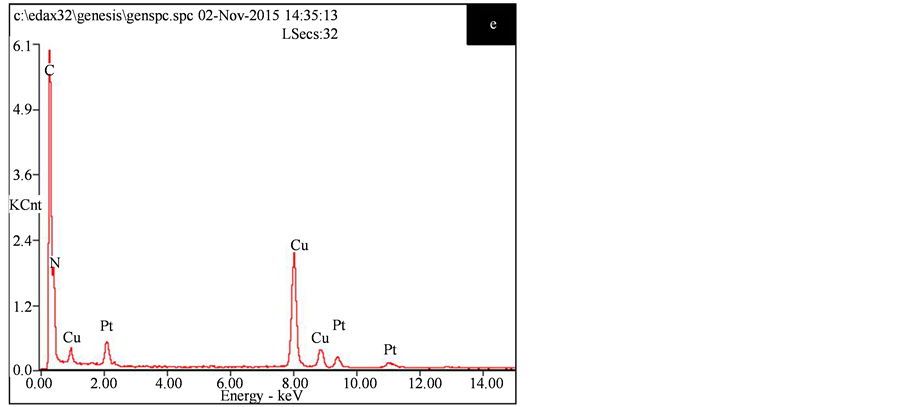
Figure 5. SEM images of the g-C3N4-550˚C (a), TEM images of the g-C3N4-550˚C (b) and the catalyst 4.2 wt.% Pt/g-C3N4-550˚C ((c), (d)), the EDX energy spectra of catalyst 4.2 wt.% Pt/g-C3N4-550˚C (e)
图5. (a)、(b)分别为g-C3N4-550℃的SEM、TEM图,(c)、(d)为4.2 wt.% Pt/g-C3N4-550℃的TEM图,(e)为4.2 wt.% Pt/g-C3N4-550℃的EDX能谱
米粒子晶格间距为0.226 nm对应于金属铂的(111)面。文献报道Pt(111)晶面更有利于C=O的吸附 [9] 。图5(e)为此催化剂的EDX能谱,证实样品含C、N、Pt元素,Cu则来自制样时所用铜网。
图6为不同煅烧温度所制备g-C3N4的FT-IR光谱图,可以发现450℃~600℃煅烧都能形成g-C3N4,与XRD表征结果一致。1200~1650 cm−1范围出现氮化碳结构中典型的CN伸缩振动吸收:1410、1462、1576和1638 cm−1处为碳氮杂环C-N和C=N的伸缩振动特征峰,1241和1321 cm−1处吸收分别归属于桥联N中C-N(-C)-C和C-N(-H)-C的伸缩振动。此外,801 cm−1处出现三嗪环的特征吸收峰 [10] [11] ,3300 cm−1处吸收则可归属为样品中残留氨基的N-H振动峰。
一般来讲,样品经过高温煅烧,其结晶度会增加,比表面积则随处理温度的增加而降低,但图7结

Figure 6. FT-IR spectrum of g-C3N4 support prepared from different calcination temperature
图6. 不同煅烧温度制备g-C3N4的FT-IR光谱图
果显示,当处理温度从450℃升到600℃时,氮化碳的比表面积从14.6 m2/g升高到了108 m2/g,这可能是不同温度下,尿素煅烧得到g-C3N4的反应路径、机理和动力学差异所致。到目前为止尿素合成g-C3N4的机理并没有明确报道,600℃煅烧形成样品的比表面积要比以往选用三聚氰胺所合成的样品(10.0 m2/g)高很多 [12] 。
催化剂4.2 wt.% Pt/g-C3N4-550℃的XPS谱如图8所示。结合能数据都用C1s标准结合能(284.8 eV)进行了校准。图8(a)的全谱扫描显示,催化剂含有C、N、Pt及少量O,其表面可能存在少量含氧物种。在图8(b)的C1s高分辨谱中,通过拟合分峰在287.0 eV和290.7 eV处得到两个特征峰,分别对应于N=C-N键中C的结合能和g-C3N4的离域π电子。图8(c)为N1s高分辨谱,在401.2 eV和403 eV处出现两个特征峰,分别归属为季氮、g-C3N4中CN杂环的离域π电子 [13] 。图8(d)为Pt4f高分辨谱,由图可知铂可能存在两种价态,Pt0和Pt2+,Pt4f7/2结合能为70.5 eV,Pt2+4f7/2的结合能为71.5 eV左右。Pt2+的存在表明负载后的Pt纳米粒子被部分氧化,生成PtO或者是Pt(OH)2 [14] 。通过计算可知Pt0质量分数为58.3%,Pt2+的质量分数为41.7%。
3.2. Pt/g-C3N4对肉桂醛选择性加氢反应的催化作用
煅烧温度的影响
制备g-C3N4所用煅烧温度对肉桂醛选择性加氢的影响列于表1。由表1可见,随着g-C3N4制备时煅烧温度从450℃升高到550℃,相同条件反应2小时后肉桂醛的转化率从15.6%提高到30%,而肉桂醇的选择性微降至65.6%。当煅烧温度升高到600℃时,肉桂醛的转化率只有26%,肉桂醇的选择性为52%,而氢化肉桂醇选择性一直保持在相对比较高的值。上述结果表明,与商业化的铂碳相比,g-C3N4负载的铂催化剂,肉桂醛转化率和肉桂醇选择性均较低,而完全加氢产物选择性相对较高。这可能是由于含氮量较高,载体表面碱性太大,与在催化反应溶剂中加入碱性试剂产生相似的结果,OH−1以某种方式与C=C结合,从而降低了C=O键的选择;另外,可能由于光诱导电子从价带(VB)过渡到导带(CB),留下同样数量的空穴,然后,激发的电子和空穴移动到催化剂表面,而激发的电子和空穴分别与吸收的电子供体和电子受体反应。但是,g-C3N4作为一种聚合物半导体材料,在没有光照或电场的作用下,达不到激发产生的电子–空穴对的能量,因此电子无法完成转移过程和表面/界面反应将能量转换为化学能,导致肉桂醛的转化率较低。由于g-C3N4多应用于光催化领域,在催化加氢方面的工作报道较少,不饱和醇选
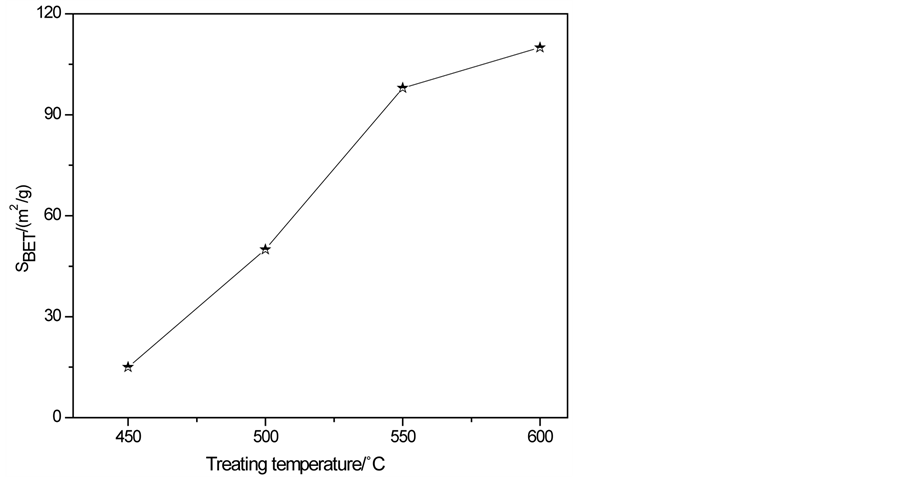
Figure 7. Effect of calcination temperature on SBET of g-C3N4
图7. 煅烧温度对g-C3N4比表面积的影响
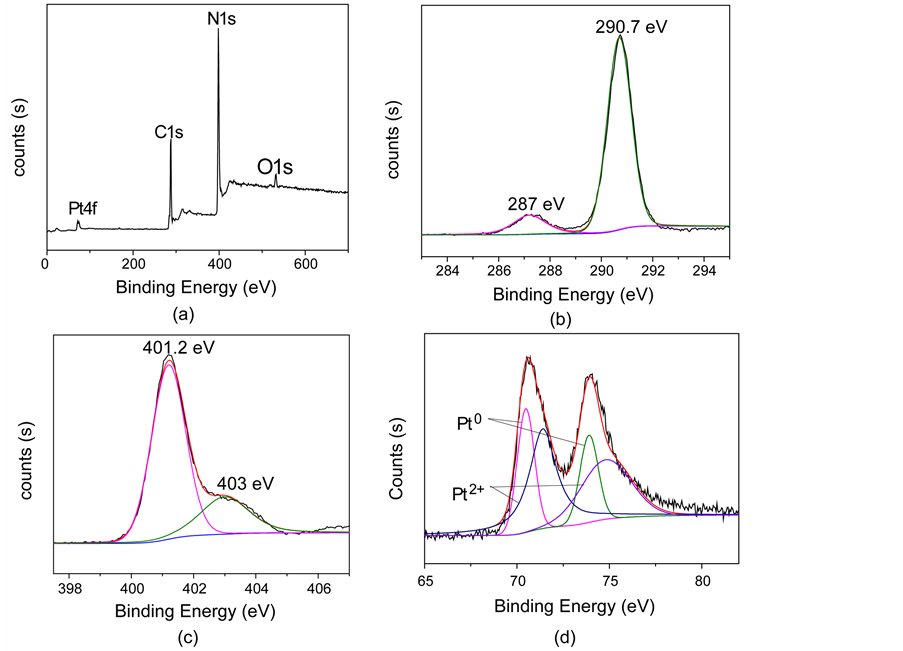
Figure 8. XPS spectra of 4.2 wt.% Pt/g-C3N4 catalysts: (a) wide scan, (b) C1s, (c) N1s, and (d) Pt4f
图8. 催化剂4.2 wt.% Pt/g-C3N4的XPS扫描光谱,(a)为全谱扫描,(b)、(c)、(d)分别为C1s、N1s和Pt4f的高分辨谱图

Table 1. Effects of samples obtained under different calcination temperatures on selective hydrogenation of cinnamaldehyde
表1. 不同煅烧温度所得样品对肉桂醛选择性加氢的影响
反应条件:0.1 g 4.2% Pt/g-C3N4,2 mL水,16 mL乙醇,2 mL肉桂醛,氢气压力:1.6 MPa,温度:80℃,转速:1000 r/min,反应时间:2 h。
表2. 4.2 wt.% Pt/g-C3N4-550℃催化剂循环使用性能的考察
反应条件:0.1 g 4.2 wt.% Pt/g-C3N4,2 mL水,16 mL乙醇,2 mL肉桂醛,氢气压力:1.6 MPa,温度:80℃,转速:1000 r/min,反应时间:2 h。
择性较低的原因目前还不清楚。
表2为4.2 wt.% Pt/g-C3N4-550℃催化剂循环使用性能考察结果。每次反应完成后,催化剂都以去离子水和乙醇分别洗涤若干次,70℃真空干燥后用于下一轮探针反应。从表中发现,使用3次后,催化剂活性基本没有变化,肉桂醛转化率和肉桂醇选择性分别保持在30%和66%左右。使用第4次时活性有所下降,但肉桂醇的选择性并没有太大变化,这主要与催化剂在使用和提纯过程中部分损失及表面吸附了少量的反应物与产物有关。
4. 结论
1) 将尿素在高温下聚合,制备了片层状g-C3N4载体,负载Pt后可得Pt/g-C3N4催化剂。
2) g-C3N4含有大量含N基团,有利于负载并稳定粒径较小、分散较好的Pt纳米粒子。
3) 载体煅烧温度对催化剂的择性加氢性能有明显影响,在较温和的条件下,催化剂4.2 wt.% Pt/g- C3N4-550℃表现出较明显的活性,肉桂醛转化率为30%,肉桂醇选择性为66%左右。重复使用3次,催化剂活性基本不变,具有良好的稳定性。
*通讯作者。