1. 引言
近年来,由于纳米金属特殊的物理化学特性引起各国科学家的浓厚兴趣。与单一的纳米金属相比,纳米双金属材料具有更大的发展前景 [1] [2] 。双金属纳米粒子是由两种不同种类的相容性材料用物理、化学方法合成的具有多相结构的双金属复合材料 [3] 。由于有些金属资源匮乏,使得我们在工业中受到限制。我们可以利用贵金属和非贵金属元素组成合金或金属件化合物,降低工业成本 [4] 。目前,科学家们已经利用水热法、模板法、多元醇还原法、共沉淀法等方法制备出了Au-Ag [5] 、Cu-Ag [6] 、Au-Pd [7] 、Ag-Pd [8] 等双金属复合纳米材料。双金属与其对应的单金属相比,有明显的电学 [9] 、光学性能 [10] [11] 的差异。
在电子科技高度发展的今天,电子元器件向微型化、低成本、多功能、高可靠性等方面发展,对电子工业用材提出了更高的要求。纳米铜和纳米银由于其自身的特性已被人们广泛应用于导电胶 [12] [13] 、导电涂料、电极材料及抗菌材料 [14] [15] 的制备等。但单独的铜和银都有一定的缺点,如单质铜容易被氧化,而银的价格昂贵,限制其在工业上的应用。我们将铜和银结合起来制备成铜银双金属,则在很大程度上克服了铜的缺点 [16] ,也降低了银的成本。并且研究与单质铜和银不同的特殊性能。
在本文中,我们提出在室温下利用绿色环保的方法,以β-环糊精作为表面活性剂,制备铜银双金属纳米材料。对反应时间对产品的影响进行了分析讨论。最后对产品的光学性能和电化学性能进行了研究。
2. 实验部分
2.1. 实验材料
硝酸银(AgNO3, ≥99%)、乙酸铜(Cu(CH3COO)2·H2O, ≥99%)、抗坏血酸(C6H8O6, ≥99%)、无水乙醇(C2H5OH, ≥99.7%)和β-环糊精等化学试剂购自重庆川东化工有限公司,均为分析纯;实验中用到的去离子水(18 MΩ)为实验室自制。
2.2. 样品的制备
本实验在乙二醇水溶液中,利用抗坏血酸还原硝酸银和乙酸铜制备铜银双金属纳米材料。其主要制备流程如图1所示:首先称取0.35 g Cu(C2H3O2)2·H2O (乙酸铜),0.3 g AgNO3 (硝酸银)和0.2 g β-CD (β-

Figure 1. The preparation procedure of Cu-Ag
图1. Cu-Ag双金属纳米材料的制备过程
环糊精),将其加入20 ml乙二醇与10 ml去离子水的混合液中,配置成铜盐与银盐的混合液;然后将混合液在室温下搅拌30 min,使得固体完全溶解;与此同时,称取0.9 g抗坏血酸溶于20 ml乙二醇溶液,并使其充分溶解;最后将20 ml的抗坏血酸溶液添加到含有Cu2+、Ag+的混合液中,使其在室温下搅拌反应1 h。通过用去离子水和乙醇在高速离心机中离心、清洗得到银灰色的Cu-Ag双金属产物(图1中红球代表Cu原子,蓝色代表Ag离子。)。最后将清洗干净的产物在烘箱中50℃干燥12 h,得到纯净的Cu-Ag双金属纳米颗粒。根据元素周期表可知,Ag+氧化性大于Cu2+氧化性,故在Ag+与Cu2+的混合体系中,Ag+优先被还原成Ag。然后以Ag为晶种,在Ag上生长出Cu-Ag双金属材料。
2.3. 表征方法
采用X射线衍射仪(XD-3,北京浦肯野通用仪器有限公司)对制得的Cu-Ag双金属纳米粉末进行了X射线粉末衍射(XRD)表征,分析条件为:以Cu Kα射线(λ = 1.54178 nm)为靶材,管电压为40 kV、管电流为200 mA、扫描速度为4˚/min,扫描的衍射角范围为20˚~80˚ (2θ);将制得的样品分散在硅片上,用Hitachi S4800型场发射扫描电子显微镜对样品进行扫描(FESEM)和能量色散X射线能谱(EDS)分析。利用X射线光电子能谱分析仪(XPS, VG ESCALAB 250 spectrometer型)测试Cu-Ag双金属纳米材料的表面数据;采用Shimadzu U-2550型紫外–可见分光光度计测试样品的紫外–可见吸收光谱(UV-vis);最后利用chi760e型号(上海辰华仪器公司)的电化学工作站分析铜银双金属纳米材料的电化学性能。
3. 结果与讨论
在室温制备Cu-Ag双金属的过程中,Cu(C2H3O2)2·H2O (乙酸铜)和AgNO3 (硝酸银)作为Cu-Ag的前驱体,抗坏血酸作为还原剂,在添加了β-CD (β-环糊精)的乙二醇水溶液中将Cu2+和Ag+还原为Cu-Ag双金属纳米材料。此体系具体离子反应机理简图如图2所示,其主要化学反应如下:
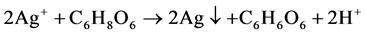
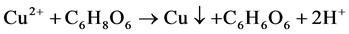
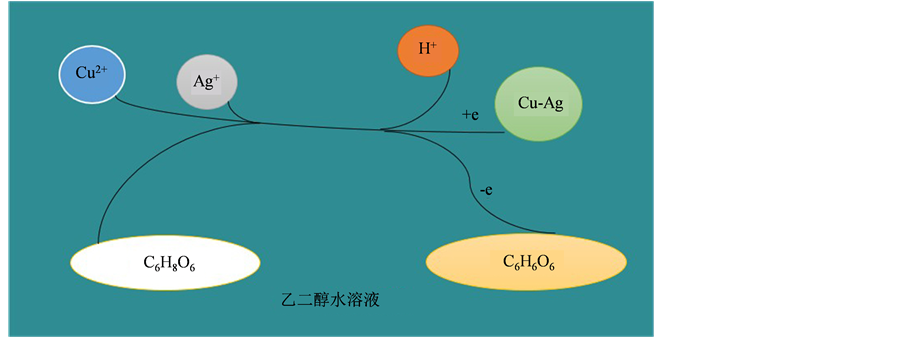
Figure 2. Ionic reaction process of Cu-Ag synthesis
图2. Cu-Ag双金属材料的离子反应机理
3.1. X射线粉末衍射(XRD)分析
利用X射线衍射分析在室温下搅拌反应1 h产生的Cu-Ag双金属纳米材料的成分和晶体结构,图3是Cu-Ag双金属纳米粒子的XRD衍射图谱。由XRD图片我们可得知产物是纯净的Cu-Ag双金属纳米材料,没有其他明显的杂质峰出现。通过查询JCPDS卡片,2θ在38.116˚,44.277˚,64.426˚和77.472˚时,分别对应着立方结构的Ag (JCPDS 04-0783)的(111),(200),(220),(311)四个晶面。当2θ为43.297˚,50.433˚和74.130˚时,分别对应着立方结构的Cu的(111),(200)和(220)晶面。在Cu-Ag双金属的XRD图谱中我们可以清晰的看到,Ag的(111)晶面是最强峰,由此可知Cu-Ag双金属中Ag的(111)晶面是晶体中最多的晶面。X射线粉末衍射的结果可以证明,Cu-Ag双金属纳米材料结晶度良好并与JCPDS卡片完全一致的。
3.2. 场发射扫描电子显微镜(FESEM)及能谱(EDS)分析
场发射扫描电子显微镜具有很高的分辨率,能清晰的看到产物的形貌结构。它对于固体材料尤其是纳米材料有非常明显的帮助。图4是室温下,不同反应时间制备的Cu-Ag双金属纳米材料的FESEM形貌图片,从图中可以看出Cu-Ag双金属晶体尺寸大约在60~200 nm范围内。并随着反应时间的延长,尺寸小于100 nm的Cu-Ag双金属晶体慢慢减少,尺寸大于100 nm的晶体数量逐渐增多(图5)。由此可知,随着反应时间的延长,Cu-Ag双金属粒子由于重结晶使得彼此融合,形成不规则的团聚物,进而造成Cu-Ag双金属材料形貌发生了很大的变化。这个现象与小晶粒最终融合成大晶粒的趋势保持一致。然后,通过Ostwald Ripening过程 [17] ,小粒子溶解进入溶液,作为大粒子生长的材料。显而易见,Cu-Ag双金属的形成是由热力学控制的过程 [18] 。图6是反应时间为1 h时产生的Cu-Ag双金属材料的FESEM-EDS图片,通过FESEM-EDS可以看出产物中主要的元素Ag、Cu和O的分布现象。观察可知Ag、Cu和O的分布区域与Cu-Ag双金属粒子的分布区域完全一致,并且没有明显的分离区。O原子的出现可能是少量的Cu被氧化,这需得进一步验证。
3.3. X射线光电子能谱(XPS)分析
我们利用X射线光电子能谱分析(XPS)测试了Cu-Ag双金属纳米材料的表面数据。图7是Cu-Ag双金属的XPS图片,我们对图谱进行分析得出,产物表面含有Ag,Cu和CuO。分析图7 XPS图谱中的峰值我们发现,Cu-Ag双金属主要的峰值有Ag的3d,Cu的2p和CuO的2p,Cu有可能被氧化成CuO。

Figure 3. XRD pattern of the sample synthesized after reaction for 1 h
图3. 室温下反应1小时后制得样品的X射线衍射图样
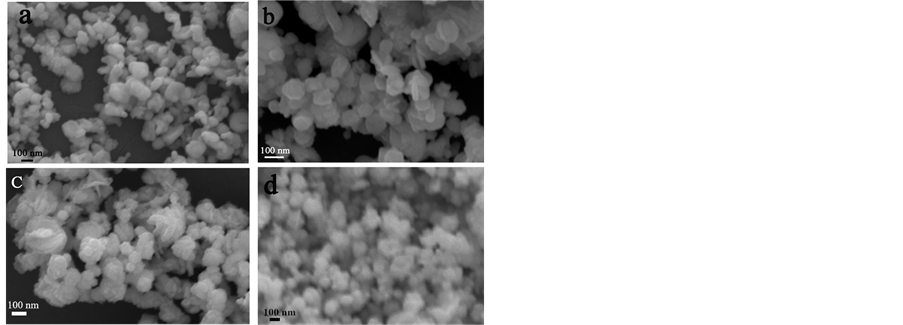
Figure 4. FESEM images of the as-prepared Cu-Ag nanoparticles with different reaction times: (a) 1 h; (b) 2 h; (c) 3 h; (d) 5 h
图4. 不同反应时间的Cu-Ag双金属的场发射图像:(a) 1小时;(b) 2小时;(c) 3小时;(d) 5小时

Figure 5. The variation trend of particle size with reaction time
图5. Cu-Ag双金属粒子尺寸随反应时间改变的变化趋势

Figure 6. FESEM-EDS images of as-prepared Cu-Ag nanoparticles for 1 h at room temperature
图6. 室温下反应1小时的Cu-Ag双金属材料的FESEM-EDS图谱
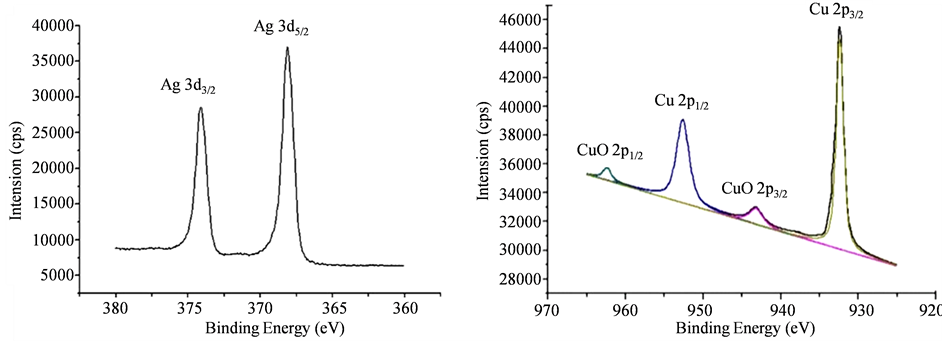
Figure 7. XPS spectra in the Ag (3d), Cu (2p) and CuO (2p) spectrum for Cu-Ag nanoparticles
图7. Cu-Ag双金属材料的XPS图谱
图谱中可分析出,Ag的3d5/2和Ag 3d3/2这两个峰值分别位于368 eV和374 eV [19] 。除此之外,Ag的峰值对称出现,进一步说明Ag的价态没有改变。Cu的峰值主要出现在零价Cu的位置,即Cu2p3/2 (932 eV)和Cu2p1/2 (952 eV)。对数据进行分析后,我们在943 eV和962 eV处得出两个小峰,其位置与CuO的峰值完全对应 [20] 。对Cu-Ag双金属材料进行XRD分析时,并没有检测出CuO的成分,这可能是由于CuO的含量特别少的原因。双金属产物的XPS详细信息如表1所示。上述数据与产物的XRD结果完全一致。由此我们可推断出Cu-Ag双金属材料可能的微观结构如图8所示。
3.4. 紫外–可见吸收光谱(UV-Vis)分析
图9是在乙醇水溶液中不同反应时间的Cu-Ag双金属材料的紫外-可见吸收光谱图像。通过图谱分析我们发现Cu-Ag双金属纳米材料仅有一个明显的吸收峰,并且随着反应时间的延长,吸收峰向短波方向移动。由此我们可以判断,产物并不是Ag纳米粒子和Cu纳米粒子的机械混合,而是在原子结构上的结合 [21] 。此外,纳米粒子的吸收峰还受其形貌和结晶度的影响 [22] 。

Table 1. XPS data for bimetallic Cu-Ag nanoparticles
表1. Cu-Ag双金属材料的XPS

Figure 8. Schematic illustration for the microstructure of bimetallic Cu-Ag
图8. Cu-Ag双金属材料可能的微观结构

Figure 9. UV-visible absorption spectra of the as-obtained bimetallic Cu-Ag with different reaction time
图9. 不同反应时间制备的Cu-Ag双金属产物的紫外分光光谱
3.5. 电化学性能分析
为了探测Cu-Ag双金属材料的电化学性能,我们首先将裸玻碳电极进行机械研磨并用Al2O3粉抛光至镜面,用酸、碱和蒸馏水彻底清洗干净,将制好的Cu-Ag双金属材料离心分离、洗涤数次,在乙醇中超声分散后滴于玻碳电极上自然风干,再以Cu-Ag修饰的玻碳电极作为工作电极,饱和Ag/AgCl电极为参比电极,Pt片电极为辅助电极,组成三电极体系。利用上海辰华仪器公司的CHI 760e型电化学工作站测定Cu-Ag双金属材料的电化学性能。
我们将不同反应时间的Cu-Ag双金属材料制成工作电极,在−0.4 V~+0.4 V的电位扫描范围内,扫描速率在50 mV/s条件下,利用上海辰华仪器公司的CHI 760e型电化学工作站测定Cu-Ag双金属纳米材料在1 mol∙L−1的Na2SO4电解液中的循环伏安曲线。图10是不同反应时间制备的Cu-Ag双金属纳米粒子的循环伏安曲线。从图中可以看出,不同反应时间的Cu-Ag双金属的CV曲线不同,并随着反应时间的变化,呈现一定的变化趋势。随着反应时间的延长,产物的峰电流逐渐减小,即Cu-Ag双金属材料的电化学活性降低。在反应时间为1 h的时候,产生的Cu-Ag双金属材料电化学活性最好。通过观测分析Cu-Ag/GC作为工作电极时的循环伏安曲线,我们发现阳极的氧化峰大概出现在0.3 V的位置,这说明了Cu-Ag双金属离子对Na2SO4有电化学活性,并进一步验证了CuO的存在 [23] 。

Figure 10. Cyclic voltammograms of Cu-Ag with different reaction time modified glassy carbon electrode in 1 mol∙L−1 Na2SO4 solution
图10. 不同反应时间的Cu-Ag双金属粒子的循环伏安曲线
图11显示了1 mol∙L−1的Na2SO4的电解液中不同反应时间的Cu-Ag双金属纳米粒子的电化学析氢反应(HER),由图12可明显看出不同反应时间产生的Cu-Ag双金属纳米粒子对极化电位有明显影响。与5 h产生的Cu-Ag双金属的析氢反应的起始过电位相比,1 h产生的Cu-Ag双金属材料的析氢反应的电位明显正移。反应时间越短,电位越正,电流密度越高。显然,不同的反应时间产生的Cu-Ag双金属纳米粒子在1 mol∙L−1的Na2SO4的电解液中的析氢活性是不同的,随着反应时间的减少,电位越正,电流密度越高,这就意味着Cu-Ag双金属纳米粒子的电催化动力学速率不断加快。研究分析得出反应时间为1 h时,Cu-Ag双金属材料的析氢性能最好。
电化学阻抗谱(Electrochemical impedance spectroscopy,简称EIS)同其他电化学测量方法一样,进行电化学阻抗谱测量的最终目的,也是要确定电极反应的历程和动力学机理,并测定反应历程中的电极基本过程的动力学参数或某些物理参数。EIS能够随时对修饰的电极材料的氧化还原分子对的电子转移阻抗进行分析 [24] 。分析产物的EIS的样品与测试CV的样品制备方式完全一致。我们测试了反应时间1 h,Cu与Ag比例为1:1的条件下的Cu-Ag双金属纳米粒子的EIS,并对其数据进行分析。其数据结果是根据测量得到的交流阻抗数据绘制的EIS谱图,我们采用曲线拟合方法对EIS图谱进行分析。拟合曲线采用R(Q(R(QR)))等效电路,恒相位角元件(Q2)和极化电阻(RP)并联,并联后再与电解液电阻(RS2)串联,此串联复合电路再与恒相位角元件(Q1)并联,最后再将已得的整个电路与电解液电阻(RS1)串联。从表2的拟合结果中发现,拟合曲线和实验数据几乎重合,卡方值小于1*10−3,达到了5.167*10−4,说明此等效电路图12中的阻抗谱的拟合结果真实可信。
4. 结论
本研究,在乙二醇的室温水溶液中,利用抗坏血酸还原硝酸银和乙酸铜制备出粒径在60~200 nm的Cu-Ag双金属纳米粒子。并通过XRD、FESEM、EDS、XPS等仪器对制备出的双金属材料进行了表征,探索了其形貌结构和内部结构组成。通过测试不同反应时间的紫外分光光谱,研究了铜银双金属材料的光学性质。最后利用电化学工作站对产物进行了CV、LSV、EIS等测试,研究了Cu-Ag双金属纳米材料在电化学方面的应用。本研究的制备方法简单、环保,产物具有良好的工业应用前景。

Figure 11. LSV curves of Cu-Ag with different reaction time
图11. 不同反应时间产生的Cu-Ag双金属材料的线性扫描曲线

Figure 12. Nyquist plots of the EIS recorded in the aq. Na2SO4 (1 mol∙L−1) for the Cu-Ag/GC
图12. 1 h反应时间下Cu-Ag双金属材料的EIS

Table 2. EIS data of the Cu-Ag nanoparticle
表2. Cu-Ag双金属粒子EIS的数据
致谢
感谢重庆市自然科学重点基金(cstc2012jjB50011),中央高校基础研究重点基金(XDJK2013B017)和重庆市基础和先进科学研究项目(cstc2013jcyjA50015, cstc2012gjhz90002)对本文的资金支持。
*通讯作者。