1. 引言
肝癌为常见的恶性肿瘤之一,严重威胁人类健康 [1] 。目前临床用于治疗肝癌的药物多为细胞毒性药物,选择性差,毒副作用大;此外,多数抗肿瘤药物为疏水性药物,难以跨越生理屏障到达肿瘤细胞,严重影响治疗效果 [2] 。纳米递送系统可提高药物的体内稳定性和选择性,增强抗肿瘤功效,降低毒副作用 [3] 。海藻酸生物相容、生物可降解、无免疫原性,富含活性羧基和羟基,易于功能化修饰,已广泛应用于药物递送 [4] [5] 。海藻酸骨架引入长链烷基、芳基等疏水性基团制得两亲性聚合物,溶液中可自组装形成纳米粒包载难溶性药物,作为纳米药物递送系统 [6] 。根据不同类型的肿瘤组织特点,通过受体介导、抗原-抗体特异性相互作用可实现对肿瘤细胞的主动靶向 [7] [8] 。肝癌细胞表面的去唾液酸糖蛋白受体可特异性识别半乳糖基团 [9] [10] ,故半乳糖修饰的纳米粒可主动靶向肝细胞。
本试验以海藻酸为亲水骨架、十六醇为疏水组分、半乳糖(Gal)为肝癌靶向分子制备两亲性聚合物,水中可自组装形成纳米粒(图1)。以DOX为模型药物,研究不同Gal接枝比HA-Gal纳米粒理化性质,释药规律和肿瘤细胞生长抑制功效。
2. 仪器和试剂
Advance DMX 500型核磁共振波谱仪(400 MHz,德国Bruker公司);VEGA TS 5136MM型可变真空扫描电子显微镜(捷克TESCAN公司);TGA 1型热重分析仪(瑞士Mettler Toledo公司);Zetasizer Nano ZS90型激光粒度仪(英国Malvern公司);Varioskan Flash 3001型酶标仪(美国Thermofisher公司);Nexus 470型傅立叶变换红外光谱仪(美国Nicolet公司);Scientz-II D型超声波细胞粉碎机(宁波新芝生物科技股份有限公司);HP6890(PLUS)型气象色谱仪(美国HP公司)。
十二烷基苯磺酸钠(SDS)、海藻酸钠(sodium alginate, NaAlg)、十六醇、4-二甲基氨基吡啶(4-(N,N- dimethylamino)pyridine, DMAP)、二甲基甲酰胺(dimethylformamide, DMF)、4-甲苯磺酸(p-toluenesulfonic acid, TSA) (国药化学试剂有限公司);阿霉素(doxorubicin hydrochloride, DOX·HCl) (浙江海正药业股份有限公司);1-乙基-3-(3-二甲氨丙基)碳二亚胺盐酸盐(N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride, EDC·HCl)、N-羟基琥珀酰亚胺(N-Hydroxysuccinimide, NHS) (上海源聚生物科技有限公司);

Figure 1. Synthetic route of HA-Gal copolymers and schematic representation depicting the formation of DOX/HA-Gal nanoparticle
图1. DOX/HA-Gal纳米粒的制备过程示意图
半乳糖胺盐酸盐(D-(+)-Galactosaminehydrochloride, Gal)、噻唑蓝(3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl Tetrazolium Bromide, MTT) (美国Sigma公司);透析袋(MWCO 3.5 kDa,上海绿鸟科技发展有限公司)。
QGY-7703细胞(人肝癌细胞,中国科学院上海生命科学研究院)。
3. 方法与结果
3.1. 两亲性海藻酸酯(HA)的制备
HA的制备方法参照文献 [6] :称取0.5 g NaAlg和0.5 g TSA,加至35 ml DMF,55℃搅拌30 min,依次加入0.3 g EDC、3.0 g十六醇,55℃反应30 h;加入4倍体积乙醇,室温搅拌30 min,8000 r/min离心20 min得到沉淀,将沉淀用无水乙醇洗涤三次,离心,溶解,透析3 d,冻干得产物HA。图2为NaAlg和HA的热重分析(Thermogravimetric Analysis, TGA)曲线图。
由图可知:NaAlg和HA均表现为两阶段失重过程,第一阶段为50℃~180℃,这主要为分子中吸附和结合水脱除所致。NaAlg第二阶段失重从216℃开始,并持续到270℃,为羧基热分解脱去CO2及相邻羟基脱水造成;相比NaAlg,HA失重更明显,是由于酯键受热分解释放CO2、相邻羟基脱去水分子,同时侧链受热分解成小分子,说明十六醇成功接枝至NaAlg。
3.2. 半乳糖(Gal)修饰的HA (HA-Gal)制备
将40 mg HA溶于10 ml去离子水,分别加入4、8和16 mg半乳糖胺,加适量的EDC和NHS (n(EDC): n(NHS):n(半乳糖胺) = 1.2:1.2:1),于室温反应24 h,透析、冷冻干燥,分别命名为HA-Gal1、HA-Gal2和

Figure 2. The TG analysis curves of NaAlg and HA
图2. 标准试验系统结果曲线
HA-Gal3。图3为NaAlg、HA、HA-Gal1、HA-Gal2和HA-Gal3傅里叶变换红外(Fourier Transform Infrared Spectrometer, FT-IR)图谱。
与NaAlg谱线相比,HA谱线中出现了一些新的吸收峰,1686 cm−1和1296 cm−1处的小尖峰为酯基中的C=O和C-O伸缩振动峰,说明HA合成。与HA相比,由于HA-Gal中N-H的伸缩振动,HA-Gal 3430 cm−1处的宽峰发生红移;1610 cm−1处的强峰为C=O和酰胺I相互叠加的结果。
图4为HA、HA-Gal1、HA-Gal2和HA-Gal3的1H-NMR图谱。3.6~5.1 ppm处的信号峰为NaAlg的氢峰。HA图谱中1.6~2.0 ppm处为HA侧链上亚甲基氢峰,1.0~1.3 ppm处为HA侧链上甲基氢峰,亦说明HA合成。HA-Gal图谱3.0~4.0 ppm处为半乳糖胺的特征峰。配制一系列标准浓度的Gal甲苯溶液,通过气相色谱测定不同浓度溶液的峰面积,然后进行线性拟合,得到Gal的标准曲线(检测器:FID,毛细管色谱柱:HP-INNOWAX,30 m ×
0.25 mm ×
0.25mm。汽化室温度:250℃,检测器温度:280℃,用高纯氮(99.99%)作为载气。色谱柱初温120℃,保持1 min,然后,以10℃/min的升温速率升至230℃,保持3 min。分流比:10:1,进样量:1 ml)。分别称取10 mg HA-Gal样品溶解在1 mol/L的NaOH溶液中,60℃下搅拌24 h然后加入适量甲苯,再充分搅拌1 h,3000 rpm分离,移取上层有机相,重复三次,将上层液进行气相色谱分析。根据气相色谱得到的峰面积计算出甲苯中Gal的浓度,最后计算得到HA-Gal1、HA-Gal2和HA-Gal3的Gal接枝比分别为3.4%、7.8%和11.4% [11] 。
3.3. 纳米粒的制备和表征
HA-Gal水中可自组装形成以十六醇为疏水核心、海藻酸和半乳糖为亲水外壳的核-壳结构纳米粒,疏水核心有利于包载难溶性药物。分别取2 ml (2 mg/ml) HA、HA-Gal1、HA-Gal2和HA-Gal3溶液,超声(120 W) 15 min,得纳米粒悬液,加入80 ml (10 mg/ml) DOX溶液,超声(120 W) 15 min,即得载DOX纳米粒悬液,分别记为DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3。分别离心,收集上清,测定吸光值,计算DOX包封率(encapsulation efficiency, EE)和载药量(loading capacity,LC,质量分数)。各载药纳米粒粒径、Zeta电势、EE和LC见表1。
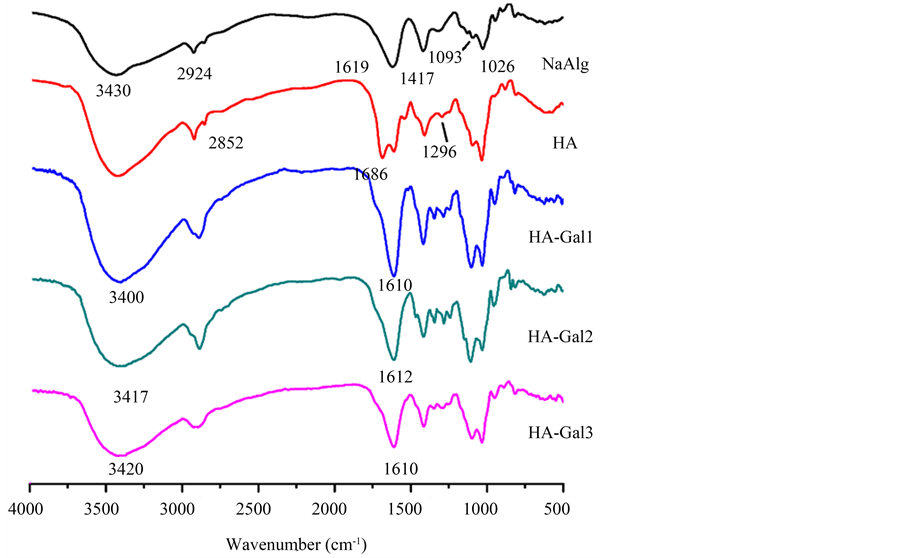
Figure 3. FT-IR spectra of NaAlg, HA, HA-Gal1, HA-Gal2 and HA-Gal3
图3. NaAlg、HA、HA-Gal1、HA-Gal2和HA-Gal3的FT-IR图谱
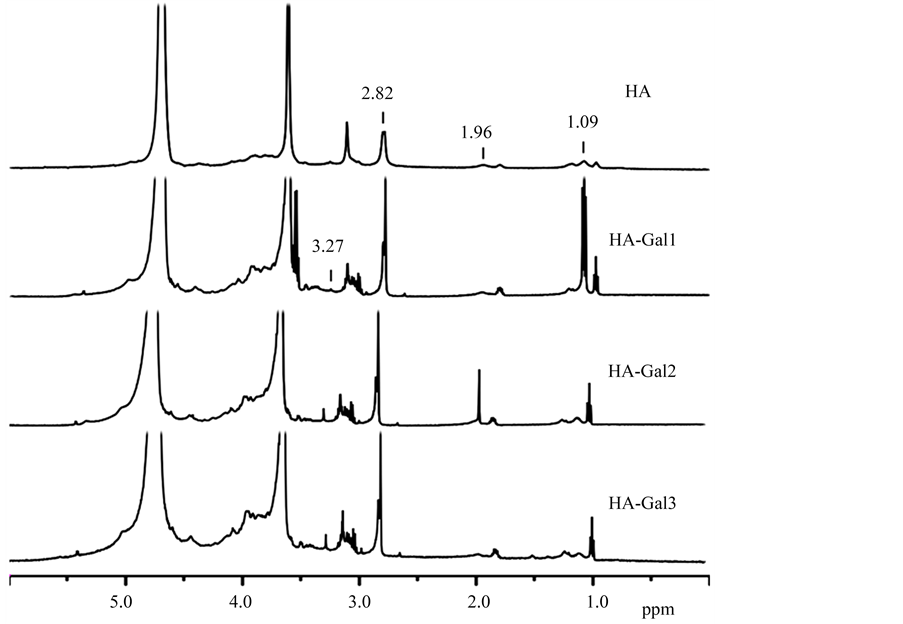
Figure 4. 1H NMR spectra of HA, HA-Gal1, HA-Gal2 and HA-Gal3
图4. HA、HA-Gal1、HA-Gal2和HA-Gal3的1H NMR图谱

Table 1. Particle size, Zeta potentials, EE and LC of DOX/HA, DOX/HA-Gal1, DOX/HA-Gal2 and DOX/HA-Gal3 (, n = 3)
表1. DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3的平均粒径、Zeta电势、EE和LC ( , n = 3)
, n = 3)
由表可知,随着Gal接枝比的增大,纳米粒粒径增大,这可能由于Gal修饰使表面亲水层增厚 [12] ;同时Zeta电势增加,可能由于海藻酸骨架游离的羧基随Gal接枝比增大而减少。以扫描电镜(scanning electron microscope, SEM)观察其形貌,可见DOX/HA-Gal2成球形,大小约为200 nm (图5)。
3.4. 体外释放
分别量取1 ml 1 mg/ml DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3纳米粒悬液(按“3.3”项下方法制备),加至透析袋中(MWCO 3500 Da),浸入50 ml 0.2 mol/mL PBS (pH 7.4),37℃、100 r/min振荡温育。分别于0.5、1、2、4、6、8、10、12、24和48 h取样1 ml,测定荧光强度。每次取样后补加1 ml新鲜PBS。由标准曲线得DOX含量,并计算累计释放量(%)。如图6所示,DOX物理包埋于纳米粒后的释药行为呈二相性,即包括初期突释和后期的缓释;前期的突释可能为少量游离及纳米粒表面粘附的DOX快速释放所致,DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3的DOX 48 h累计释放分别达到80%、82%、88%和93%。
3.5. 细胞摄取
按2.3项下方法制备含DOX的纳米粒。以1 × 105细胞/孔的密度接种QGY-7703细胞至24孔板,37℃、5% CO2培养24 h;每3孔为一组,按每孔5 mg DOX加入纳米粒,37℃、5% CO2分别培养0.5、2和4 h;吸弃孔内液体,以0.2 mol/L PBS (pH 7.4)洗涤三次,加入0.5 ml 0.5% (w/v) SDS溶液(pH 8.0),37℃、100 r/min振荡培养30 min以裂解细胞;酶标仪测定细胞裂解液中DOX含量,Lowry法测定蛋白质含量。摄取量以每1 mg蛋白质含DOX的量(mg)表示(mg/mg)。
纳米粒粘附至细胞表面后会被细胞内化,摄取入胞,细胞摄取效率和药物递送效率正相关 [13] 。
图7为QGY-7703细胞对纳米粒的摄取试验结果,可见在QGY-7703细胞中,纳米粒在0.5~4 h内可提高药物的细胞摄取量,且摄取量随Gal接枝比的增加而增加,其可能为靶向配体Gal修饰度和纳米粒的粒径、电势等物理性质共同作用的结果。
3.6. 细胞毒性试验
以1 × 104细胞/孔的密度接种QGY-7703细胞至96孔板,37℃、5% CO2培养24 h;更换新鲜培养基,每3孔为一组,加入终浓度分别为25、50、250和500 mg/ml的空白纳米粒,以加等体积0.2 mol/L PBS (pH 7.4)为对照,37℃、5% CO2培养48 h;MTT法测定细胞存活率。
图8为QGY-7703细胞与不同浓度空白纳米粒共培养48 h的存活率,可见在25~500 mg/ml浓度范围内纳米粒对细胞活力无显著影响(p > 0.05),可确保后续体外细胞试验结果的准确性。

Figure 5. SEM images of DOX/HA-Gal2
图5. DOX/HA-Gal2的SEM照片

Figure 6. In vitro release of DOX/HA, DOX/HA-Gal1, DOX/HA-Gal2 and DOX/HA- Gal3 under neutral (pH 7.4) condition at 37˚C (n = 3)
图6. DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3体外释放曲线(n = 3)

Figure 7. Cellular uptake of DOX in QGY-7703 cells following incubation for 0.5, 2, and 4 h at 37˚C (n = 3)
图7. QGY-7703细胞对纳米粒的摄取(n = 3)
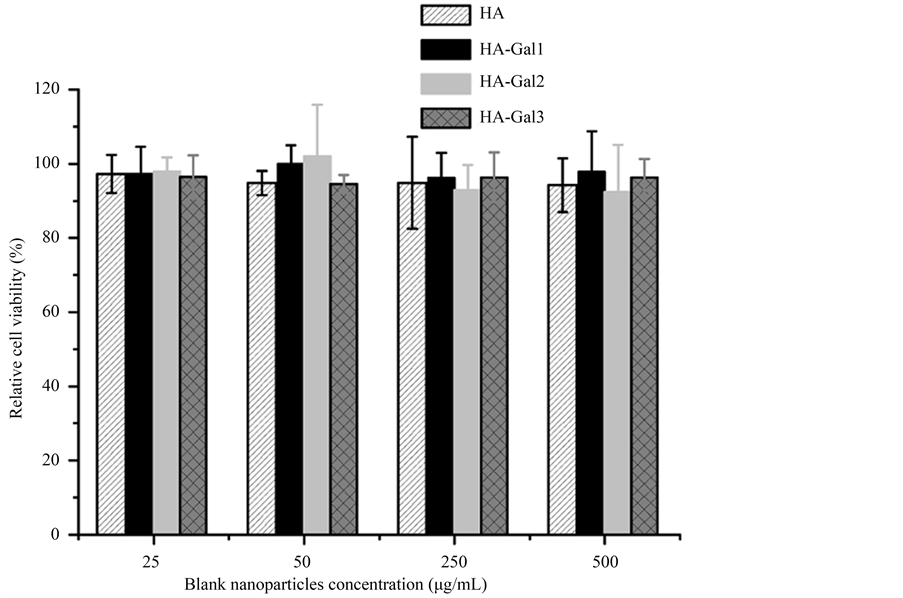
Figure 8. Viability of QGY-7703 cells after incubation with blank nanoparticles at various concentrations for 48 h (n = 3)
图8. QGY-7703细胞分别与空白纳米粒共培养48 h后的细胞存活率(n = 3)
3.7. 体外细胞抑制
按2.6项下培养细胞,每6孔为一组,分别加入适量游离DOX、DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3纳米粒悬液,使孔内DOX终浓度分别为0.1、0.2、0.5、1、2和5 mg/ml,以加入0.2 mol/L PBS (pH 7.4)的细胞为阴性对照,37℃、5% CO2培养48 h;用MTT法测定各试验组相对细胞存活率,计算半数抑制浓度(IC50)。
图9为QGY-7703细胞分别与游离DOX溶液和纳米粒共培养48 h后的细胞存活率,可见细胞存活率随药物浓度升高而降低。DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3组的细胞存活率显著低于游离DOX组,表明纳米粒可增强药物的肿瘤细胞抑制作用。游离DOX、DOX/HA、DOX/HA-Gal1、DOX/HA-Gal2和DOX/HA-Gal3的IC50分别为1.12、0.68、0.64、0.51和0.46 mg/ml,表明体外抗增殖能力随着靶向配体接枝比的增加而增强,与细胞摄取结果一致,纳米粒提高了DOX的肿瘤细胞摄取效率,更多药物入胞,抑制肿瘤细胞增殖。
4. 讨论
用十六醇对海藻酸进行修饰,制备得到两亲性HA,半乳糖胺的氨基与HA的羧基缩合形成酰胺键,制得HA-Gal,HA-Gal水中自组装形成纳米粒,超声条件下包载抗肿瘤药物DOX,得到具有纳米尺度的DOX/HA-Gal。该纳米粒水化后粒径约为200 nm,具有良好的单分散性,随Gal接枝比的增加,纳米粒表面靶向配体与受体的结合能力增强,通过特异性受体介导的内吞作用主动转运入胞,实现纳米粒的主动靶向,同时Zeta电势绝对值降低,与荷负电的细胞表面静电排斥减弱,更有利于细胞的摄取,更多药物进入胞内,抑制肿瘤细胞增殖。此外,Gal修饰度高的纳米粒药物释放更快,高效入胞后,胞内药物浓度提高,更有利于实现高效的细胞抑制。总之DOX/HA-Gal3具有更强的肿瘤细胞增殖抑制功效,其可归因于表面靶向配体修饰度、纳米粒自身粒径、电势和药物释放等理化性质共同作用的结果,HA-Gal3纳米粒有望作为理想的靶向抗肿瘤药物递送载体用于肿瘤治疗。

Figure 9. Viability of QGY-7703 cells after incubation with drug loaded nanoparticles at various concentrations for 48 h (n = 6)
图9. QGY-7703细胞分别载药纳米粒共培养48 h后的细胞存活率(n = 6)
*通讯作者。