1. 引言
铜的氧化物包括氧化亚铜(Cu2O)和氧化铜(CuO),通常为p型半导体,禁带宽度Eg分别为1.9~2.2 eV和1.2 eV [1] - [4] ,能够吸收可见光,具有可见光光催化活性 [1] [4] - [7] 。是目前被广泛研究的金属氧化物半导体光催化材料之一。
黄铜是常见的铜锌合金,在溶液中能够发生化学或电化学腐蚀 [8] 。在不同的电解质溶液中电化学阳极氧化黄铜时还可以在样品表面得到不同的氧化膜 [9] - [14] 。Morales [11] 等人的研究表明黄铜经过在硼酸盐-硼酸缓冲液和0.5 mol∙L−1氯化钠 + 硼酸硼酸盐缓冲液中阳极处理后能产生富铜的钝化层,具有耐局部腐蚀性。Biton [12] 等用恒电位方法研究了黄铜在高浓度KOH溶液中的阳极氧化膜,表明在较低电位(−350 mV vs. SHE)下黄铜表面主要为Cu2O,在较高电位(−150 mV vs. SHE)下则形成Cu2O/Cu(OH)2双层结构。Maryam [14] 等用报道了在0.01~0.2 mol∙L−1 NaOH溶液中,黄铜经过6~24 V的电位阳极氧化后,通过控制电解液浓度、电压和阳极氧化时间,可以得到纳米至微米尺寸的球状、花状、多孔、片状、八面体和立方体形状的表面氧化膜。但是以往的研究主要集中在黄铜阳极氧化膜的生成过程和防腐蚀应用方面,尚未有光电催化性能的研究报道。为了拓展黄铜材料在光电催化方面的应用,本文通过在NaOH溶液中恒电流阳极氧化,在黄铜表面构建氧化膜,分析测试了氧化膜的光电性能及结构、性能,探讨了NaOH浓度等条件对黄铜薄膜阳极氧化膜形貌、结构和光电化学性能的影响。
2. 实验
2.1. 黄铜表面氧化薄膜的制备
以市售的H70黄铜(α-黄铜,Cu:Zn 70:30)为阳极,纯铜片(99.99%)为阴极,采用二电极体系对黄铜进行阳极氧化。黄铜基底经过机械打磨、超声清洗、乙醇和蒸馏水清洗处理后,在55℃的1~10 mol∙L−1的NaOH电解液中,10 mA∙cm−2的阳极电流密度极化300 s。固定阴阳极的面积比和间距分别为4:1和2.0 cm。经阳极氧化后的黄铜片经纯水、乙醇清洗后置入马弗炉中,在空气气氛下,以5℃∙min−1升温至150℃,恒温加热2 h,再自然冷却至室温。实验中所用试剂均为分析纯试剂。
2.2. 薄膜物相和形貌表征
采用荷兰Phillips公司的PW3040/60型X射线衍射(XRD)分析薄膜的结构。通过日本Hitachi公司的S4800型扫描电子显微镜(SEM)分析薄膜表面形貌。用英国Renishaw RW1000型显微共焦激光拉曼光谱仪对样品进行拉曼光谱检测,激发波长为514.5 nm。用机械剥离法将氧化膜从基底上剥离后,在日本电子公司的JEM.2100F型透射电子显微镜上进行TEM表征。
2.3. 氧化物薄膜的光电性能测试
采用两电极体系,以经阳极氧化的黄铜片为工作电极,样品受光面积为2 cm2,Pt片为对电极,电解液为0.1 mol∙L−1 Na2SO4。光源为Xe灯(北京纽比特科技有限公司)并使用紫外截止滤光片ZJB380滤去紫外光部分。在零偏压下测试样品的光电流~时间(j~t)曲线。所有的电化学/光电化学测试均在CHI660C电化学工作站上进行。
3. 结果与讨论
3.1. NaOH浓度对氧化膜形貌、结构的影响
图1所示为不同NaOH浓度下制备的样品表面的SEM图像。可见在相同的阳极氧化电流和时间条件下,在1.0 mol∙L−1 NaOH溶液中得到的氧化膜为数十纳米的颗粒(图1(a))。NaOH浓度为3.0~5.0 mol∙L−1时则得到纳米片(图1(b)和图1(c)),尺寸约数百纳米,边缘不规则。NaOH浓度继续升高至8.0~10.0 mol∙L−1,样品表面又变成尺寸不均一的多面体颗粒,但与在1.0 mol∙L−1 NaOH溶液得到样品相比,颗粒尺寸更加不均匀。
图2所示为不同NaOH浓度下制备的样品的XRD图谱。所有的样品中都出现2θ为42.324˚、49.274˚、72.243˚和87.452˚的四个强峰,分别对应于基底的α-黄铜相(111)、(200)、(220)和(311)晶面的衍射峰(JCPDS NO.50-1333)。经阳极氧化后,出现了一些新的衍射峰,这些衍射峰很弱,表明样品表面生成的氧化膜非常薄。但可以清楚分辨出在1.0~5.0 mol∙L−1 NaOH溶液中阳极氧化后的样品(图2(a)~(c)),在32.563˚、35.604˚、38.712˚和67.985˚处出现CuO(110)、(200)、(111)和(113)晶面的衍射峰(JCPDS NO. 80-1268),在29.521˚、36.507˚和61.908˚出现Cu2O(110)、(111)和(220)晶面的衍射峰(JCPDS NO. 74-1230)。而在8和10 mol∙L−1 NaOH溶液中阳极氧化的样品,32.563˚、35.604˚、38.712˚和67.985˚的衍射峰基本消失,29.521˚和61.908˚的衍射峰明显增强,并且在84.337˚出现对应于Cu2O(321)晶面的衍射峰(图2(d)、图2(e))。可见,当其它实验条件不变时,在1.0~5.0 mol∙L−1 NaOH溶液中阳极氧化的样品,表面氧化膜中主要存在Cu2O和CuO。而在8.0~10 mol∙L−1 NaOH溶液中阳极氧化的样品,表面只有Cu2O。在XRD谱图中没有出现ZnO的衍射峰,这应该是由于强碱性条件下,黄铜中的Zn被氧化后转变成了可溶性的锌酸盐类物种而扩散进入液相,固相氧化膜不含ZnO。
图3给出了样品的表面Raman散射分析结果。NaOH浓度为1.0~5.0 mol∙L−1时阳极氧化的表面膜在300、348、630和1133 cm−1处出现Raman散射峰,浓度为8.0~10.0 mol∙L−1时的氧化膜则在150、221、297、417、513、649和789 cm−1处出现Raman散射峰。Hamilton [15] 等人通过原位Raman研究了NaOH溶液中铜的阳极氧化膜,将147、220、297、412、492、515、609、649、661和786 cm−1处的峰归属于Cu2O的Raman散射峰,而指认300、347和635 cm−1的峰为CuO的Raman散射峰。Hagemann [16] 等人则报道了单晶CuO在1100~1200 cm−1之间存在Raman峰。可推知在1.0~5.0 mol∙L−1 NaOH溶液中得到的氧化膜主要为CuO,但在140和230 cm−1弱峰显示有少量的Cu2O存在,8.0~10.0 mol∙L−1 NaOH溶液中得到的氧化膜表面主要为Cu2O。所有样品中也没有检测出ZnO的存在。在1.0~5.0 mol∙L−1 NaOH中得到

Figure 1. Morphologies of the anodic oxide films on the brass grown in various NaOH concentration (mol/L, 55˚C) :(a) 1.0; (b) 3.0; (c) 5.0; (d) 8.0; (e) 10.0
图1. 不同NaOH浓度下黄铜阳极氧化膜的表面形貌:NaOH 浓度(mol/L,55℃): (a) 1.0;(b) 3.0;(c) 5.0;(d) 8.0;(e) 10.0
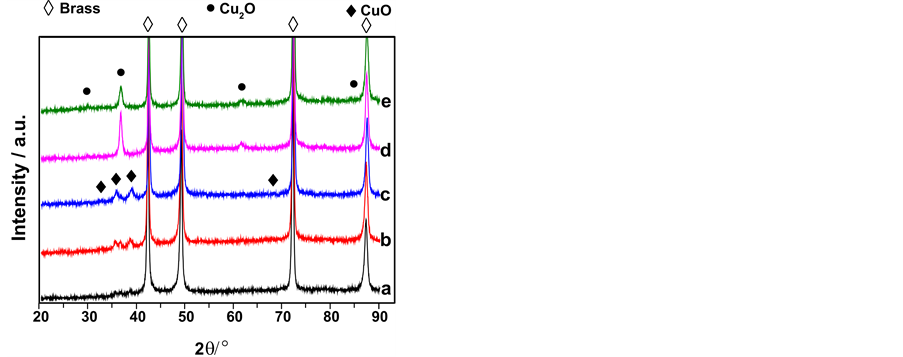
Figure 2. XRD pattern of the anodic oxide films on the brass grown in various NaOH concentration(mol/L, 55˚C): (a) 1.0 (×5); (b) 3.0 (×5); (c) 5.0 (×5); (d) 8.0; (e) 10.0
图2. 不同NaOH 浓度下黄铜阳极氧化膜的XRD 图谱:NaOH 浓度(mol/L,55℃):(a) 1.0;(b) 3.0;(c) 5.0;(d) 8.0;(e) 10.0
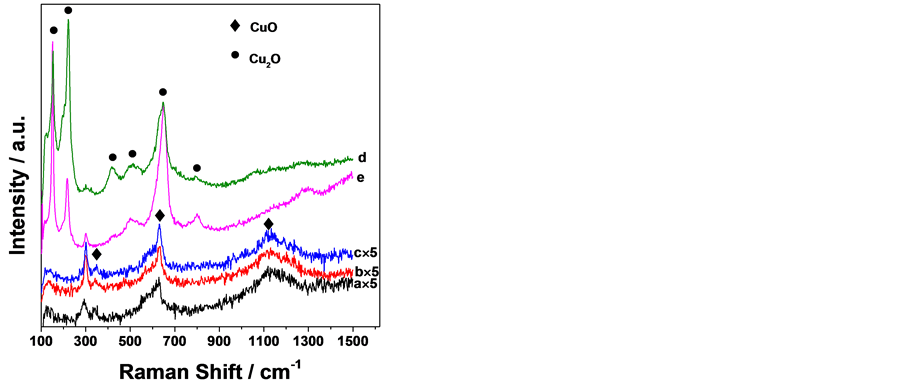
Figure 3. Raman spectra of the anodic oxide films on the brass grown in various NaOH concentration (mol/L, 55˚C): (a) 1.0 (×5); (b) 3.0 (×5); (c) 5.0 (×5); (d) 8.0; (e) 10.0
图3. 不同 NaOH 浓度下黄铜阳极氧化膜的Raman 散射图谱:NaOH 浓度(mol/L,55℃):(a) 1.0 (×5); (b) 3.0 (×5);(c) 5.0 (×5);(d) 8.0;(e) 10.0
的氧化膜没有出现明显的Cu2O Raman散射峰,与XRD分析显示的明确存在Cu2O的结果不完全一致,可能与此条件下的氧化膜具有Cu2O为底层而CuO在表层的双层结构以及表面Raman分析的检测深度比XRD浅有关 [12] 。
综合SEM、XRD和Raman的分析结果,可以看到,在其它制备条件都相同时,随着NaOH浓度从1.0 mol∙L−1增加到10 mol∙L−1,黄铜表面的氧化膜形貌和组成都发生明显的变化。在NaOH浓度为3.0和5.0 mol∙L−1时,氧化膜为纳米片状的Cu2O/CuO结构。TEM分析进一步表明,纳米片上存在很多无序堆积的纳米棒。这些纳米棒长度为数十纳米(图4)。La [17] 等在碱性溶液中阳极氧化纯铜的研究结果揭示,在20℃的1.0 mol∙L−1 NaOH溶液中,阳极氧化得到沿着[100]晶向优先取向生长的斜方相Cu(OH)2纳米束,当电解液温度高于50℃时,Cu(OH)2形成之后会发生后续的脱水过程形成纳米片状CuO。在黄铜阳极氧化过程中形成氧化物纳米片的过程可能与此类似。但详细的形成机理及阳极氧化参数的影响,还需进一步研究。
3.2. 薄膜光电性能分析
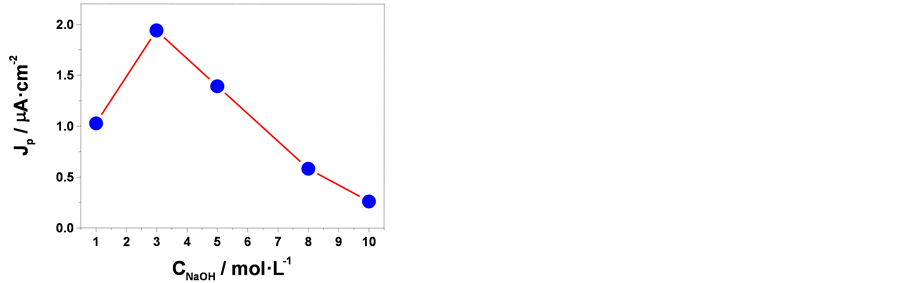
在含0.1 mol∙L−1 Na2SO4的电解液,用两电极体系,在零偏压情况下测试了样品的光电化学性能。图5(a)是在3.0 mol∙L−1 NaOH溶液中阳极氧化得到的样品在可见光照射下产生的光电流响应曲线。从图中可以看到,在开始光照的瞬间,出现明显的阴极光电流,然后逐渐降低,最终出现~2 μA∙cm−2的阴极电流平台。阴零偏压下出现阴极光电流是p型半导体的典型特征 [18] 。当光源被遮住时阴极光电流立即消失,电流密度趋于零。所有经阳极氧化后得到的样品都有类似的光电响应行为。我们取无光和有光条件下的相邻电流平台的电流差值并取绝对值,以多次光电流测试结果的平均值作为衡量样品光电化学性能的指标。在电流密度为10 mA∙cm−2,阳极氧化时间为300 s,电解液温度为55℃,退火条件为150℃ 2 h的条件下,不同NaOH浓度中制备的样品的光电流密度总结在图5(b)中。
从图5(b)中可以看出,在1.0~10.0 mol∙L−1的范围内,随着NaOH浓度的升高光电流密度值先增大后减小。在NaOH浓度为3.0~5.0 mol∙L−1时得到的阳极氧化膜显示最大的光电流(平均光电流密度分别为1.9和1.4 μA∙cm−2)。结合SEM、XRD和Raman的分析结果,可知在3.0~5.0 mol∙L−1 NaOH溶液中制得的样
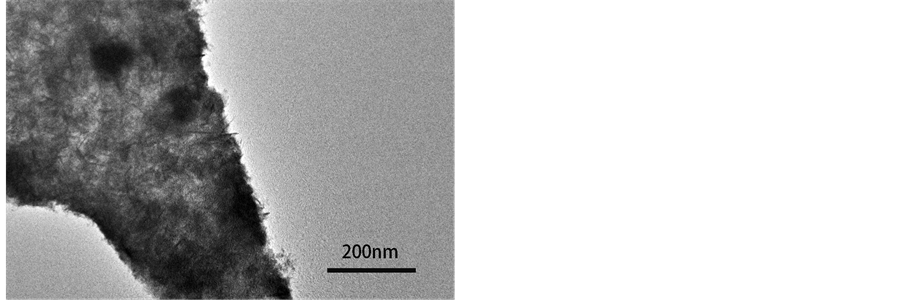
Figure 4. TEM image of nanoplate oxide obtained from 3.0 mol∙L−1 NaOH
图4. 3.0 mol/L NaOH 溶液中得到的氧化物纳米片的TEM图像
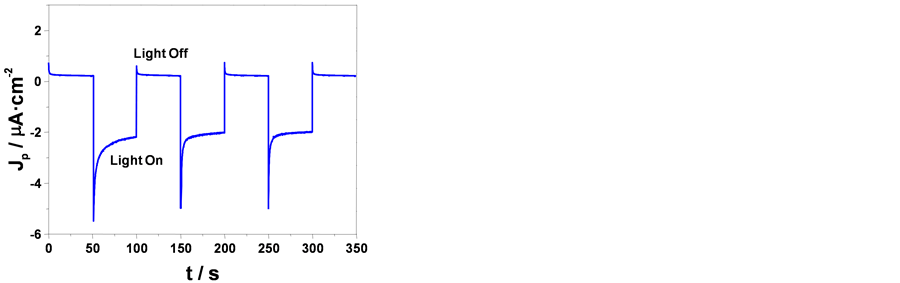
 (a) (b)
(a) (b)
Figure 5. (a) The photocurrent-t plot of the oxide film obtained from 3.0 mol/L NaOH at zero bias potential in 0.1 mol/L Na2SO4 electrolyte under visible light; (b) the photocurrents of the oxide films obtained from various concentration NaOH solution
图5. (a) 3.0 mol∙L−1 NaOH溶液中得到的样品在零偏压下的可见光光电流–时间曲线;(b) 光电流密度值随阳极氧化时电解液中NaOH浓度变化曲线
品都是具有纳米片形貌的Cu2O/CuO复合氧化物。这些纳米片在样品表面堆积成多孔结构,使样品表面比表面积增大,可以增加光生载流子与溶液中反应物的接触几率。而且纳米片氧化物中产生的光生载流子扩散纳米片/溶液界面的路径较短,降低了光生载流子复合几率。同时多孔结构便于光子进入氧化膜深处,并能使光子在孔内多次反射,有利于提高薄膜的吸光效率。因此它们显示出良好的光电化学性能。二者在光电流密度上的差别,可能是两种氧化膜的形貌、Cu2O/CuO的相对比例及厚度有所不同所导致的。Wu [19] 等通过PVA的辅助作用两步法制备出Cu2O/CuO纳米棒薄膜,在可见光下测得Cu2O/CuO纳米棒薄膜的光电流约为6 μA∙cm−2。本文所得Cu2O/CuO纳米片样品的光电化学性能比Cu2O/CuO纳米棒差,但本文的制备过程更为简便。通过探索优化阳极氧化的参数,可望在黄铜表面制备出光电化学性能更加优异的纳米结构氧化膜。
4. 结论
1) 采用恒电流阳极氧化法,在二电极体系中电流密度为10 mA∙cm−2,时间为300 s,电解液温度为55℃的条件下,在1.0~10.0 mol∙L−1范围内改变阳极氧化时的NaOH浓度,在黄铜表面制备出不同形貌和组成的氧化物薄膜。这些氧化膜在可见光照射下都产生p型半导体的阴极光电流。
2) 在3.0~5.0 mol∙L−1 NaOH溶液中得到的氧化膜具有纳米片状的多孔形貌和Cu2O/CuO双层结构。在0.1 mol/L Na2SO4溶液中,它们在零偏压条件下的可见光光电流密度分别为1.9和1.4 μA∙cm−2。显示出较好的光电化学性能。
致谢
感谢国家自然科学基金(No 21173196)的资助。