1. 引言
自从1991年首款锂离子电池问世以来,因其放电电压平稳、自放电率低、使用寿命长、能量效率高、易充电等优点备受关注 [1] - [3] 。锂离子电池是以正极材料为锂离子嵌入化合物的一类电池的总称,它主要由正电极、负电极、电解质溶液和隔膜四个部分组成。该化合物中的锂离子可以在晶体中脱出和嵌入(外电场作用下),而且这个过程是可逆的;负极材料则通常采用的是层状结构的碳材料;电解质溶液则为溶解有可溶性锂盐的有机溶剂碳酸酯溶液(如碳酸乙烯酯(EC)、碳酸丙烯酯(PC)、碳酸二甲酯(DMC))。锂离子电池中Li+在正负电极间往返地脱出与嵌入的历程就是锂离子电池的充电和放电。因为Li+在整个过程中始终在正负电极间来回移动,所以锂离子电池被人们形象的叫做“摇椅电池”(简称RCB) [4] 。
嵌锂化合物是锂离子电池中的锂离子的“贮存库”,选择高电势的嵌锂化合物是为了获取比较高的单体电池电压。综合各方面因素,优良的正极材料应该具备如下的优点:原料成本低廉,清洁无毒,安全稳定性,与电解液和粘结剂的兼容性好,循环性能强,使用寿命长,倍率性能佳,低温性能好。
锂离子电池的正电极材料的选择将直接影响锂离子电池的清洁环保、充放电容量、安全稳定、循环、使用寿命等性能特点。当前,正电极材料的热点研究主要集中在尖晶石型LiM2O4和层状LiMO2(M = Co,Ni,Mn,V等)结构的化合物上 [5] - [10] ,但是在实用过程中也表现出了诸多问题:如不可逆容量损失、极化电压增大、结构不稳定、化学性能差、循环容量下降、价格高等,这些问题的出现极大的影响了其实用化进程,及时解决这些问题对于挖掘其潜在功能、提高效率、节约能源有着重大意义。就当前的市场化锂离子电池而言,其正极材料主要包括LiCoO2、LiNi1/3Co1/3Mn1/3O2、LiFePO4、LiXMn2O4四种产品。
1) LiCoO2,该材料是锂离子电池最常见的正极材料,生产工艺简单一般适用于各类小型电子设备,其工作电压高,循环性能好,低温下稳定性强。但是这种材料的电池安全性和抗过充电性都相对比较差,有毒,Co资源短缺,价格昂贵,还无法使用在大容量车用与储能锂离子电池的正极上。
2) LiNi1/3Co1/3 Mn1/3O2,该材料被应用于手机电池和动力电池等很多产品之中,其价格便宜,与电解液相容性好,自放电率低,清洁环保,可逆比容量高,循环性能好。但是这种材料的制作工艺比较严苛,热稳定性能差,充放电过程中容易发生结构的变化。
3) LiFePO4,该材料是近些年以来发展较快的正极材料,在动力电池与备用电源方面具有广阔而实际的运用前景。其是一种磷酸盐聚阴离子化合物,安全性能高,稳定性强,耐高温性能好,使用寿命长。但是这种材料的循环性能弱,电导率低,高倍率充放电性能差,合成中Fe离子极易氧化。
4) LiXMn2O4,该材料制备工艺相对简单,主要应用在高功率动力电池上,成本低廉,其热稳定性与抗过充电性都强过以上介绍的两种材料,无毒且清洁环保,安全性能高。但是这种材料的理论比容量低,循环性能差。
近些年以来,我国在锂离子电池行业己经逐步取代了日本成为了世界第一的生产制造国,但是关于锂离子电池的研究、开发和利用仍然远远地落后于日本与欧美这些发达国家。
基于上述分析,本研究采用溶胶凝胶法制备了LixMn3-xO4和LiFeO2嵌锂正极材料,并对系列样品的结构、磁性、电化学性能进行了研究,为提高LixMn3-xO4和LiFeO2嵌锂正极材料的容量、稳定性以及充放电能力等提供实验依据,并为尝试制备既可以作为正极材料也可作为磁存储材料的多元器件开拓了思路。
2. 实验方法
2.1. LixMn3-xO4(X = 0.7, 1.0, 1.2, 1.4, 1.6)的制备
实验采用溶胶凝胶法,所有试剂均为分析纯。按照化学计量比,称量碳酸锂(Li2CO3)加入60 ℃去离子水(500 ml)中,在磁力搅拌器不断搅拌下,滴加浓硝酸,直至溶液澄清透明而且不再有气泡产生为止。再多加两滴浓硝酸溶液,使溶液的PH值保持在3~4之间。向所得溶液中加入50%的硝酸锰(Mn(NO3)3溶液并不断搅拌。随后加入称量好的柠檬酸(C6H8O7∙H2O)和乙二醇(C2H6O2),并继续在60℃下搅拌20分钟,得到溶胶。将溶胶放在100℃的水浴锅中,加热24小时后,溶胶转变为凝胶。将得到的凝胶放入鼓风干燥箱中直接升温到90℃,每升高10℃保持半个小时,最后升到200℃,在此温度下保持12小时。将干燥好的的凝胶样品放到箱式马弗炉中直接升温至200℃,每升高50℃保持半个小时。升高至400℃,在此温度下保持5个小时。样品随炉冷却至室温后,将所得样品用玛瑙研钵进行研磨。将样品放入箱式马弗炉直接升温到700℃保持3个小时。样品随炉冷却至室温后,将所得样品用玛瑙研钵进行研磨。最后将研磨所得粉末放入箱式马弗炉中直接升温到950℃保持3个小时。样品随炉冷却至室温后,将所得样品用玛瑙研钵进行研磨。
2.2. LiFeO2的制备
按照化学计量比,称量碳酸锂(Li2CO3)粉末加入60℃去离子水(500 ml)中。在磁力搅拌器不断搅拌下,滴加浓硝酸,直至溶液澄清透明而且不再有气泡产生为止。随后向溶液中加入98.5%的硝酸铁(Fe(NO3)3)溶液并不断搅拌。随后加入称量好的柠檬酸(C6H8O7∙H2O)和乙二醇(C2H6O2),并继续在60℃下搅拌20分钟,得到溶胶。将制备所得的样品在100℃的水浴锅中,加热24小时后溶胶转变为凝胶。将得到的凝胶放在鼓风干燥箱中,直接升温到100℃烘干并在此温度下保持12小时。然后将得到的凝胶放入箱式马弗炉中直接升温到500℃,保持5个小时,冷却后用玛瑙研钵进行研磨。随后再将所得的粉体放入箱式马弗炉中直接升温到700℃,并在此温度下保持5个小时,冷却后用玛瑙研钵进行研磨。最后将获得的粉体放入箱式马弗炉中直接升温到1000℃,并在此温度下保持2个小时,冷却后用玛瑙研钵进行研磨。
2.3. 样品的表征
利用X射线衍射(XRD)测试样品的结构,物理性能综合测试系统(PPMS)用于测试样品的磁性。采用电池性能测试仪对正极材料的储电、充放电以及稳定性等性能进行测试。
电池组装过程:首先按8:1:1的比例称量样品和乙炔黑跟PVDF,将实验样品和乙炔黑混合并在浴霸灯照射下研磨30分钟左右,再加入PVDF研磨3~5分钟。然后将其涂在铝箔上拉膜,拉好的膜120℃鼓风烘箱烘干12小时左右。最后剪下电极膜的区域,辊压2次,冲制成直径14 mm的电极片,称量电极片质量,编号后放入电子干燥柜干燥,从而完成对样品电极片制备;接下来跟据叠放次序负极壳 + 锂片 + 2滴电解液 + 隔离膜 + 2滴电解液 + 正极片 + 不锈钢片 + 铝球 + 正极壳的流程(电解液为碳酸乙烯酯(EC)组装电池。
3. 结果和讨论
3.1. LixMn3-xO4(X = 0.7, 1.0, 1.2, 1.4, 1.6)和LiFeO2的结构
图1所示为LixMn3-xO4(X = 0.7, 1.0, 1.2, 1.4, 1.6)粉末的XRD谱,由图可以看出,各粉末样品的衍射峰与尖晶石结构LiMn2O4的标准谱(PDF卡片号:01-088-1030)吻合较好,均具有Fd-3m空间群结构。尖晶石结构的LiMn2O4是以O原子的立方堆积为骨架,Li离子占据四面体8a的位置,Mn离子占据八面体16d的位置,在立方晶胞中形成四面体簇。在八面体16c位形成了三维结构的共边八面体,所以通常认为[Mn2]O4阵列是密堆积的主体框架结构,其中四面体晶格和八面体晶格共面,构成了互通的三维离子通道 [11] 。此外,随着锂离子含量的增加,(111)衍射峰逐渐增强,与(311)、(400)和(511)衍射峰相比,仍然较弱,这与卡片标准谱(111)为主峰并不一致,因此,我们推测图中标记为(311)衍射峰中含有Mn3O4的(211)衍射峰,即粉末样品为LiMn2O4和Mn3O4的混合物。随着锂离子含量的增加,LiMn2O4相(111)逐渐增强,说明Mn3O4相含量逐渐减少,LiMn2O4相逐渐增加。
图2所示为LiFeO2的XRD谱,样品呈现α-NaFeO2型铁氧体结构,空间群为Fm3m,与标准谱(PDF卡片号:01-085-1991)吻合较好。利用谢乐公式对样品晶粒尺寸D进行了估算,结果表明,粉末样品的晶粒尺寸接近于100 nm,这说明样品的表面效应较弱,可以忽略表面效应对其性能的影响。
3.2. LixMn3-xO4(X = 0.7,1.0,1.2,1.4,1.6)和LiFeO2的磁性
我们对LixMn3-xO4(X = 0.7, 1.0, 1.2, 1.4, 1.6)系列样品进行了室温下磁性测试,结果显示,所有样品均显示顺磁特性,没有明显的磁滞行为。图3给出了样品Li1.2Mn1.8O4室温下的M-H曲线。
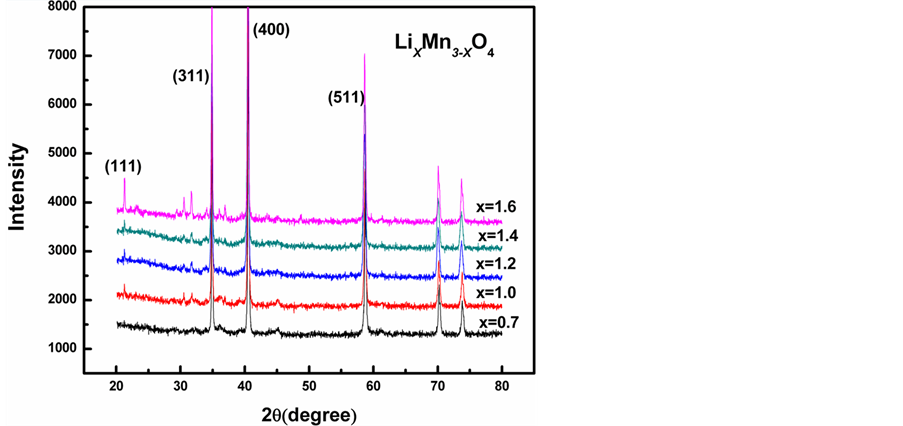
Figure 1. XRD patterns of the LiXMn3-XO4(X = 0.7, 1.0, 1.2, 1.4, 1.6)
图1. LiXMn3-XO4(X = 0.7, 1.0, 1.2, 1.4, 1.6)的XRD图谱

Figure 2. XRD pattern of the LiFeO2
图2. LiFeO2的XRD图谱

Figure 3. M-H curve of the Li1.2Mn1.8O4
图3. Li1.2Mn1.8O4的M-H曲线
图4给出了LiFeO2室温下的M-H曲线,由图中可以看到,样品呈现软磁特性,饱和磁矩为0.25 emu。我们知道,LiFeO2铁氧体具有α-NaFeO2结构,空间群为Fm3m,其中较大的氧离子以面心立方密堆积方式排列,较小的金属阳离子占据氧离子的八面体间隙位置。与文献 [12] 报道相比,Li1.067Fe0.933O2样品在10 K与404 K之间呈现顺磁性不同,我们制备的样品在室温下呈现软磁特性,这可能是由于Li离子和Fe离子磁矩在晶格中有序排列所致。
3.3. Li1.2Mn1.8O4和LiFeO2的电化学性能
Li1.2Mn1.8O4样品在0.2 C电流密度下的进行充放电测试,如图5、图6。样品的首次充、放电比容量分别为58.16 mA∙h/g和29.47 mA∙h/g,首次充放电效率为50.7%。经过101次循环后,充放电比容量分别为20.45 mA∙h/g和19.75 mA∙h/g,平均库伦效率为93%以上,循环稳定性良好。首次循环与第101次
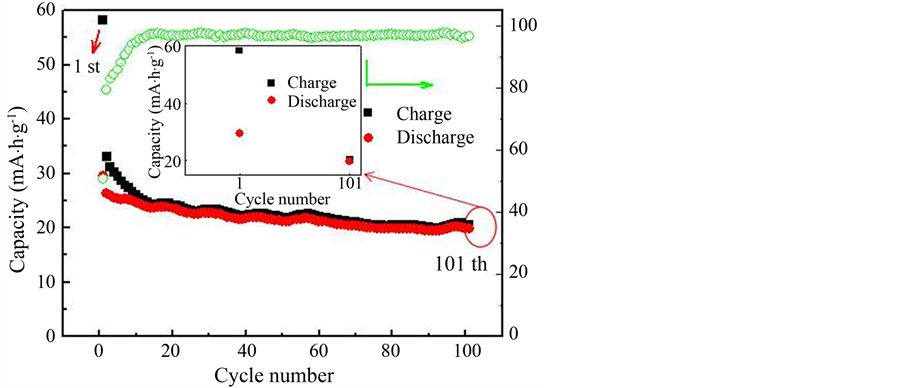
Figure 5. The charge-discharge curves of the Li1.2Mn1.8O4
图5. Li1.2Mn1.8O4样品的充放电曲线
循环的数据位置如图5中箭头,相应的数据如小图。倍率性能是衡量电池在不同电流密度下循环,所表现出的容量大小、保持率和恢复能力的性能。在倍率测试中,在低倍率电流密度0.2 C下,样品的首次充、放电比容量分别为57.66 mA∙h/g和29.33 mA∙h/g,首次充放电效率分别为51%。经过数次循环后充放电容量分别为27.38 mA∙h/g和24.56 mA∙h/g,与100次循环的性能一致。随着电流密度增大,充放电比容量逐渐降低,后面五段的第五次放电容量分别为20.98、18.17、15.45、11.66和8.66 mA∙h/g,相应的充放电效率如图中绿色图线所示,分别为95.76%、98.16%、98.42%、96.87%和96.37%。在大电流下循环,各段放电容量比较平稳,无急剧下降现象,表明电极结构稳定,能够承受大电流冲击。在第七段小电流循环中,第五次的充放电容量分别为22.68 mA∙h/g和22.02 mA∙h/g,相应的效率为97.1%。能够恢复到小电流密度下循环的性能,表明其具有较好的性能恢复能力。由于其它样品的充放电容量太小在这里不做说明,其原因正在进一步研究。
LiFeO2样品在0.2 C电流密度下的进行充放电测试,如图7、图8。样品的首次放电比容量为 38.50 mA∙h/g,经过26次循环后,放电比容量为24.46 mA∙h/g,无较大幅度的容量降低,表明其具有良好的循
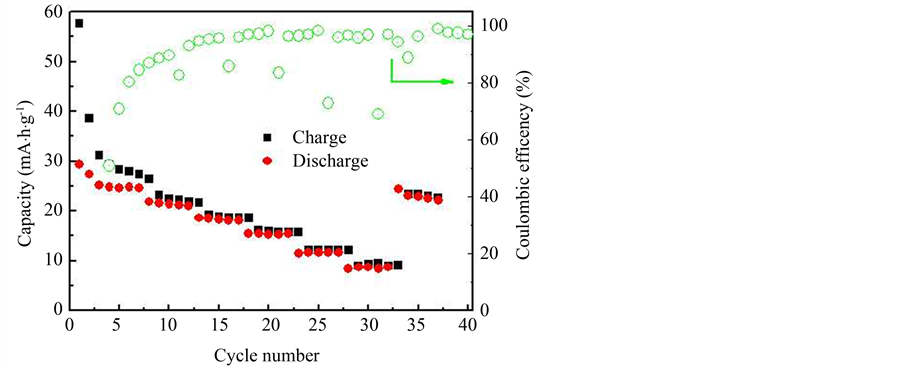
Figure 6. The rate curve of the Li1.2Mn1.8O4
图6. Li1.2Mn1.8O4样品的倍率曲线
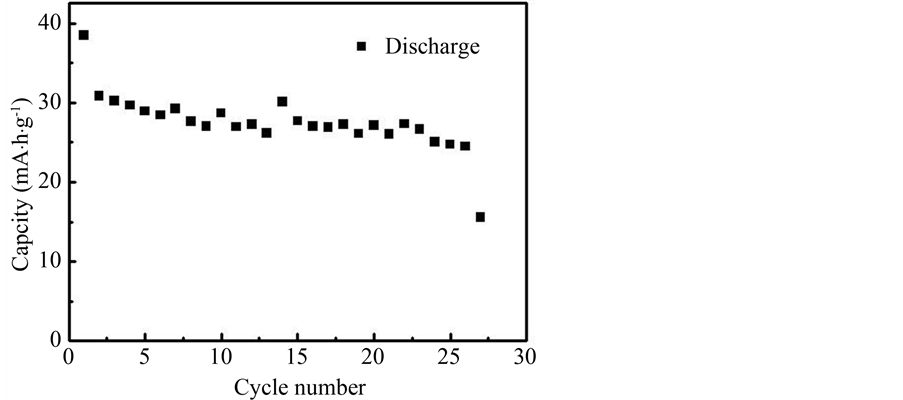
Figure 7. The charge-discharge curves of the LiFeO2
图7. LiFeO2样品的充放电曲线
环稳定性。在倍率测试中,在低倍率电流密度0.2 C下,样品的首次充、放电比容量分别为 80.95 mA∙h/g和39.18 mA∙h/g,首次充放电效率为48.4%。经过数次循环后充放电容量分别为27.01 mA∙h/g和 29.58 mA∙h/g,与前面循环的性能基本一致。随着电流密度增大,充放电比容量逐渐降低,后面五段的第五次放电容量分别为25.2、23.219、20.736、15.96和10.55 mA∙h/g,其各段的可逆容量均比Li1.2Mn1.8O4样品的各段可逆容量均高20%以上,相应的充放电效率如图8中绿色图线所示,分别为96.12%、95.8%、95.59%、93.04%和89.18%。在大电流下循环,LiFeO2样品的各段放电容量比较平稳,无急剧下降现象,表明电极结构稳定,能够承受大电流冲击。在第七段小电流循环中,第五次的充放电容量分别为22.65 mA∙h/g和25.79 mA∙h/g,相应的效率为96.76%。倍率性能方面,LiFeO2样品所表现出的性能要优于Li1.2Mn1.8O4样品,同样的测试条件下,表现出更好的耐大电流冲击,具有更好的可恢复能力。
4. 结论
本文采用溶胶凝胶法制备了LixMn3-xO4(X = 0.7, 1.0, 1.2, 1.4, 1.6)和LiFeO2锂离子电池正极材料,并

Figure 8. The rate curve of the LiFeO2
图8. LiFeO2样品的倍率曲线
对其结构、磁性、电化学性能开展了研究。实验发现,LixMn3-xO4系列样品呈现尖晶石结构,室温下显示顺磁特性,在x = 1.2的样品中,样品的首次充、放电比容量分别为58.16 mA∙h/g和29.47 mA∙h/g,首次充放电效率为50.7%。经过101次循环后,充放电比容量分别为20.45 mA∙h/g和19.75 mA∙h/g,平均库伦效率为93%以上,循环稳定性良好。LiFeO2样品为α-NaFeO2型铁氧体结构,而且室温下呈现软磁特性,在低倍率电流密度0.2 C下,样品的首次充、放电比容量分别为80.95 mA∙h/g和39.18 mA∙h/g,首次充放电效率为48.4%,经过数次循环后充放电容量分别为27.01 mA∙h/g和29.58 mA∙h/g,与前面循环的性能基本一致。本文的研究为提高嵌锂正极材料容量、稳定性以及充放电能力等性能提供了实验依据。
基金项目
感谢河北省自然科学基金(A2012101001)和河北省承德市财政局项目(CZ2012007,CZ2013002)资助。