1. 引言
没食子酸是一种天然多酚类化合物,其化学名为3, 4, 5-三羟基苯甲酸,广泛存在于五倍子、漆树和茶等植物中。没食子酸及其酯具有抗菌、抗病毒和抗肿瘤等多种生理活性 [1] - [3] 。羽扇烷醇属于五环三萜类天然产物,在自然界中含量较少。文献报道表明,羽扇烷醇没有抗肿瘤活性 [4] ,但其结构修饰物具有多种生物活性 [4] - [8] 。据文献报道,羽扇烷醇二元羧酸单酯具有抗HIV活性 [6] [7] 。笔者最近的研究表明,羽扇烷醇二元羧酸单酯对肿瘤细胞株A549、HepG2和HeLa的增殖具有很强的抑制活性,其抗肿瘤活性比阿霉素的强 [4] ;肉桂酸羽扇烷醇酯对食管鳞癌细胞Eca-109、TE-1和EC-9706的增殖均有良好的抑制活性,其抗肿瘤活性与阿霉素的相近 [8] 。目前,未见文献报道没食子酸羽扇烷醇酯。本文以羽扇烷醇和没食子酸为原料,报道了新的化合物没食子酸羽扇烷醇酯的合成方法(见图1),并以顺铂和阿霉素作阳性对照药物,研究了其对肿瘤细胞株A549、LAC和HepG2增殖的抑制活性。
2. 实验方法
2.1. 主要仪器和试剂
Brucker DRX-400核磁共振仪,TMS为内标;MDS Sciex API 2000 LC/GC/MS质谱仪;Perkin-Elmer 240C 元素分析仪;Synergy HT多功能酶标仪(美国BioTek公司);羽扇烷醇由本实验室按文献方法制备获得 [9] 。3-(4′,5′-二甲基噻唑-2′)-2,5-二苯基四氮唑溴盐(MTT) (广州博强生物科技有限公司);青霉素、链
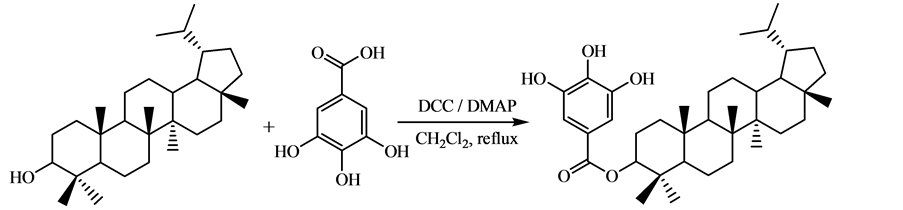
Figure 1. Synthesis of lupanol gallate
图1. 没食子酸羽扇烷醇酯的合成
霉素、PRMI-1640培养基和胎牛血清(杭州四季青生物工程材料公司);阿霉素(深圳万乐药业有限公司);顺铂(济南齐鲁制药有限公司);没食子酸(Alfa Aesar Co.);其它试剂均为分析纯。
2.2. 没食子酸羽扇烷醇酯的合成
在干净的100 mL圆底烧瓶中加入214.36 mg (0.50 mmol)羽扇烷醇、102.08 mg (0.60 mmol)没食子酸、123.80 mg (0.60 mmol) DCC、4.89 mg (0.04 mmol) DMAP和30 mL二氯甲烷,装上球形冷凝管,并在冷凝管上装一个干燥管,干燥剂为无水氯化钙,然后回流(40℃)搅拌,反应时间通过薄层层析(TLC)跟踪来确定。反应10 h后冷却,过滤,滤液减压浓缩,以石油醚:丙酮 = 4:1作洗脱剂,硅胶200~300目,残留物经柱层析得白色固体202.1 mg,产率为69.6%。产物Rf = 0.52 (展开剂:二氯甲烷);1H NMR (400 MHz, CDCl3), d:0.74 (3H, s), 0.77 (3H, s), 0.81 (3H, s), 0.85 (6H, d, J = 8.0 Hz), 0.92 (3H, s), 0.95 (3H, s), 1.01 (3H, s), 1.24~1.34 (11H, m), 1.36~1.37 (3H, m), 1.50~1.54 (3H, m), 1.63~1.66 (8H, m), 1.83~1.97 (1H, m), 4.57~4.64 (1H, m), 7.01 (2H, s), 其中3个活泼氢被氘代;ESI-MS, m/z: 579 [M-1]-. Anal. calcd for C37H56O5: C 76.51, H 9.72; found C 76.21, H 9.75。
2.3. 没食子酸羽扇烷醇酯的体外抗肿瘤活性
2.3.1. 细胞培养
人非小细胞肺癌细胞株A549、肺腺癌细胞株LAC和肝癌细胞株HepG2来源于暨南大学医药研究开发基地,它们分别在含10%胎牛血清、100 U/mL青霉素和100 μg/mL链霉素的RPMI-1640培养基 (pH 7.4)中生长。
2.3.2. 细胞毒理试验 [8]
收集对数期细胞1 × 104接种于96孔板,置于37℃,5% CO2培养箱培养使细胞贴壁。加入不同浓度的待测样品,实验组每个浓度做3个平行孔,37℃,5% CO2条件下培养72 h。加入20 μL MTT溶液(5 mg/mL,即终浓度为0.5% MTT)继续培养4 h。终止培养,吸去孔内培养液,每孔加入150 μL DMSO,置于摇床上震荡10 min,使结晶物充分溶解。在酶联免疫检测仪570 nm处测量各孔的吸光度值。实验同时设调零孔(培养基,MTT,DMSO)、对照孔 (细胞,培养液,MTT,DMSO)和阳性对照孔(顺铂或阿霉素)。计算细胞生长抑制率的公式如下:

其中A样品、A对照和A空白分别表示样品、对照和空白试验的吸光度。
2.3.3. 统计分析
使用统计软件SPSS11.5处理实验数据。每个样品对癌细胞生长的抑制试验均重复3次,其抑制率为3次实验的平均值,求得样品或药物对癌细胞生长抑制50%所需要的浓度,即是IC50。实验结果见表1。

Table 1. IC50 values of lupanol gallate against human tumor cell lines
表1. 没食子酸羽扇烷醇酯对肿瘤细胞株的IC50值
3. 结果与讨论
3.1. 没食子酸羽扇烷醇酯的合成
目前,没食子酸酯的合成方法主要有4种 [1] [2] [10] - [16] :1) 直接酯化法。没食子酸与脂肪醇在路易斯酸的作用下反应,合成没食子酸酯,产率可达90%以上。该方法主要适用于制备没食子酸脂肪醇酯。2) 羟基保护-酯化法。该方法是没食子酸通过苄基化或乙酰化反应先将其3个酚羟基保护起来,然后与二氯亚砜或五氯化磷反应形成酰氯,再与醇反应形成酯,最后脱去保护基制得没食子酸酯。该方法反应步骤较多,副反应多,产率较低,且使用有腐蚀性的酰化试剂。3) DCC法。没食子酸的3个酚羟基用苄基或乙酰基保护,然后以N,N'-二环己基碳二亚胺(DCC)作缩合剂和4-二甲氨基吡啶(DMAP)作催化剂,与醇反应形成酯,再脱去保护基制得没食子酸酯;或者以DCC作缩合剂和DMAP作催化剂,没食子酸与醇直接反应制备没食子酸酯。前者反应条件温和,反应时间短,产率高,反应底物醇的适用范围广,但反应步骤较多;后者反应条件温和,反应时间短,产率较低,反应底物醇的适用范围较窄。4) 生物酶催化合成法。在酶的催化下,没食子酸与醇反应合成没食子酸酯。寻找合适的酶作催化剂,是制备没食子酸酯的关键。目前该方法仍不成熟,产率较低。没食子酸羽扇烷醇酯是一种新化合物,其合成方法未见文献报道。本文以DCC作缩合剂,DMAP为催化剂,羽扇烷醇与过量的没食子酸在二氯甲烷中回流10 h (回流温度为40℃),一步反应合成了没食子酸羽扇烷醇酯,产率为69.6%。该方法不需要酚羟基的预先保护,操作简便。
3.2. 没食子酸羽扇烷醇酯的体外抗肿瘤活性
以非小细胞肺癌细胞A549、肺腺癌细胞LAC和肝癌细胞HepG2为肿瘤细胞模型,顺铂和阿霉素为阳性对照药物,笔者采用MTT法研究了新化合物没食子酸羽扇烷醇酯对上述3株肿瘤细胞的体外抗肿瘤活性,实验结果见表1。
样品或药物的抗肿瘤活性分别通过上述3株肿瘤细胞生长的半数抑制浓度来表达,即IC50。以IC50 < 100 μM作为评价样品或药物是否具有抗肿瘤活性的标准。IC50值越小,表示样品或药物的抗肿瘤活性越好;而IC50 >100 μM,则表示样品或药物没有抗肿瘤活性。从表1可见,顺铂和羽扇烷醇对上述3株实验肿瘤细胞株的IC50值均高于100 μM,表明它们对实验肿瘤细胞株的增殖没有抑制作用;而没食子酸羽扇烷醇酯和阿霉素对实验细胞株的增殖具有抑制作用。没食子酸羽扇烷醇酯对非小细胞肺癌细胞A549和肺腺癌细胞LAC的IC50值分别为51.71 μM和62.16 μM,其对上述2株肿瘤细胞增殖的抑制作用不及阿霉素;而没食子酸羽扇烷醇酯对肝癌细胞HepG2的IC50值为64.34 μM,其对HepG2增殖的抑制活性比阿霉素强;没食子酸羽扇烷醇酯对A549和HepG2的抗肿瘤活性比羽扇烷醇二元羧酸单酯的弱 [4] 。
4. 结论
1) 在DCC和DMAP的作用下,羽扇烷醇与过量的没食子酸在二氯甲烷中回流10 h,一步反应合成了新化合物没食子酸羽扇烷醇酯,产率为69.6%。该合成方法不需要酚羟基的预先保护,操作简便。
2) 没食子酸羽扇烷醇酯对A549和LAC增殖的抑制活性不及阿霉素,而对HepG2增殖的抑制活性比阿霉素强。
基金项目
国家级星火计划项目(2014GA780069)和广东省普通高校特色创新项目(2014KTSCX161)。