摘要:
以La(NO3)36H2O、Co(NO3)36H2O和Fe(NO3)39H2O为原料,用共沉淀法对制备LaFexCo1−xO3 (x = 0.2, 0.5, 0.8)钙钛矿进行研究。热重–差示量热分析(TG-DSC)和X射线衍射(XRD)分析表明,合成LaFexCo1−xO3钙钛矿过程有四段失重,分别在110℃之前、150℃~220℃、300℃~500℃和700℃~780℃;经过700℃~800℃煅烧后有钙钛矿形成,之后随煅烧温度提高,钙钛矿生成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃时获得钙钛矿的量最多,但是与800℃和900℃煅烧后形成钙钛矿相比,其晶粒尺寸增加较多;随着Fe取代Co的量增加,形成钙钛矿尺寸晶粒尺寸逐渐降低。
Abstract:
Preparation of LaFexCo1−xO3 (x = 0.2, 0.5, 0.8) perovskite by coprecipitation using La(NO3)36H2O, Co(NO3)36H2O和Fe(NO3)39H2O and Fe(NO3)39H2O as raw materials was researched. The phenomena observed were characterized using Thermogravimetry-Differential Scanning Calorimetric (TG-DSC) and X- ray diffraction (XRD). Results showed that process of preparing LaFexCo1−xO3 perovskite appeared four period of weight loss at less than 110˚C, 150˚C - 220˚C, 300˚C - 500˚C and 700˚C - 780˚C, respectively. The sample calcined at 700˚C - 800˚C has already formed perovskite, then amount of forming perovsktie and the grain size of perovskite increase gradually with the calcination temperature. When calcination temperature is at 1000˚C, the most amount of perovskite is gained, but the grain size increases more than that of perovskite calcined at 800˚C and 900˚C. With the increase of substitute Fe for Co, perovskite particle size is gradually reduced.
1. 引言
钙钛矿的通式是ABO3,其中A位是半径较大的金属阳离子,通常为碱金属、碱土金属或稀土金属;B位是离子半径较小的金属阳离子,通常为过渡金属离子 [1] [2] 。多数情况下钙钛矿ABO3型氧化物中的A、B位离子的价态不是固定的,只要价态之和等于6,并且离子半径符合容限因子的计算公式,均有可能形成钙钛矿化合物 [2] [3] 。钙钛矿型复合氧化物的A、B位可以同时容纳多种不同的金属离子,形成 或
或 复合氧化物。从热力学平衡角度来看,这些元素的混合价态往往不够稳定,有时只需在较小的能量驱动下即可发生电子结构匹配上的转变,从而获得优良的磁学、光学、电学、表面和催化性能,使其在高温超导、离子导电、催化剂等领域得到较好的应用 [2] [3] 。目前,研究者已经采用采用氧化物高温烧结法、盐分解法、溶胶–凝胶法和共沉淀法等成功制备出钙钛矿。其中,氧化物高温烧结法具有烧结温度高,煅烧时间长,且形成的复合氧化物往往含有杂质相、颗粒比较大、容易烧结、比表面积小、催化性能差 [4] [5] ;盐分解法具有设备简单、操作简便、混合比较均勾、煅烧温度低于固相法的优点,但是产品需要的锻烧时间长、比表面积小、催化性能不理想 [6] [7] ;溶胶–凝胶法具有颗粒细、比较面积大、合成温度低等优点,但是所需原材料价格较贵、颗粒比较容易出现团聚、凝胶过程比较耗时耗能 [8] [9] [10] [11] 。而共沉淀法则是制备含有两种或两种以上金属元素的复合氧化物超细粉体的重要方法,反应物之间的混合是离子级别的,沉淀后各组分达到分子级的均匀混合,烧结后得到氧化物晶型比较单一,但是与溶胶凝胶法相比,制备的产品颗粒尺寸稍微偏大 [12] [13] [14] 。
复合氧化物。从热力学平衡角度来看,这些元素的混合价态往往不够稳定,有时只需在较小的能量驱动下即可发生电子结构匹配上的转变,从而获得优良的磁学、光学、电学、表面和催化性能,使其在高温超导、离子导电、催化剂等领域得到较好的应用 [2] [3] 。目前,研究者已经采用采用氧化物高温烧结法、盐分解法、溶胶–凝胶法和共沉淀法等成功制备出钙钛矿。其中,氧化物高温烧结法具有烧结温度高,煅烧时间长,且形成的复合氧化物往往含有杂质相、颗粒比较大、容易烧结、比表面积小、催化性能差 [4] [5] ;盐分解法具有设备简单、操作简便、混合比较均勾、煅烧温度低于固相法的优点,但是产品需要的锻烧时间长、比表面积小、催化性能不理想 [6] [7] ;溶胶–凝胶法具有颗粒细、比较面积大、合成温度低等优点,但是所需原材料价格较贵、颗粒比较容易出现团聚、凝胶过程比较耗时耗能 [8] [9] [10] [11] 。而共沉淀法则是制备含有两种或两种以上金属元素的复合氧化物超细粉体的重要方法,反应物之间的混合是离子级别的,沉淀后各组分达到分子级的均匀混合,烧结后得到氧化物晶型比较单一,但是与溶胶凝胶法相比,制备的产品颗粒尺寸稍微偏大 [12] [13] [14] 。
本文采用TG-DSC和XRD分析研究了共沉淀法制备钙钛矿 钙钛矿加热过程的变化,并探讨了不同Fe含量(x = 0.2, 0.5, 0.8)对制备钙钛矿的影响。
钙钛矿加热过程的变化,并探讨了不同Fe含量(x = 0.2, 0.5, 0.8)对制备钙钛矿的影响。
2. 试验
2.1. 样品的制备
首先称取一定量的 (≥ 99%,广州苏喏化工有限公司)、
(≥ 99%,广州苏喏化工有限公司)、 (≥ 98.5%,上海展云化工有限公司)和
(≥ 98.5%,上海展云化工有限公司)和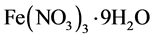 (≥ 98.5%,上海展云化工有限公司)分别加入蒸馏水溶解配置成浓度都为0.1 mol/L的水溶液,然后按照
(≥ 98.5%,上海展云化工有限公司)分别加入蒸馏水溶解配置成浓度都为0.1 mol/L的水溶液,然后按照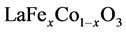 (其中x取0.2,0.5和0.8)化学计量比将相应金属硝酸盐溶液混合,然后在微波振荡混匀的过程中逐滴加入0.2 mol/L的Na2CO3 (≥ 99%,上海展云化工有限公司)和0.2 mol/L的NaOH (≥ 99.7%,广州市才允多化工贸易有限公司)混合溶液,控制溶液的pH值为10~11,1 h后将混合液经过离心分离得到沉淀和清液,倒出清液后的沉淀加入蒸馏水经过微波分散后再采用离心分离,如此反复5~7次直至离心后的上清液为中性,将所得沉淀放入烘箱中于100℃下干燥4 h,将干燥产物研磨成粉(即钙钛矿前驱体)后放入电炉中于400℃预烧2 h,最后在试验的温度下锻烧。
(其中x取0.2,0.5和0.8)化学计量比将相应金属硝酸盐溶液混合,然后在微波振荡混匀的过程中逐滴加入0.2 mol/L的Na2CO3 (≥ 99%,上海展云化工有限公司)和0.2 mol/L的NaOH (≥ 99.7%,广州市才允多化工贸易有限公司)混合溶液,控制溶液的pH值为10~11,1 h后将混合液经过离心分离得到沉淀和清液,倒出清液后的沉淀加入蒸馏水经过微波分散后再采用离心分离,如此反复5~7次直至离心后的上清液为中性,将所得沉淀放入烘箱中于100℃下干燥4 h,将干燥产物研磨成粉(即钙钛矿前驱体)后放入电炉中于400℃预烧2 h,最后在试验的温度下锻烧。
2.2. 热重–差示量热分析
采用德国的Netzsch STA409PC热分析仪对钙钛矿前驱体进行热重–差示量热分析,然后根据热重–差示量热分析结果确定锻烧的工艺条件。测试条件:升温速率为10℃·min−1,升温范围为室温~1200℃。
2.3. X射线衍射(XRD)分析
煅烧后的样品磨细后采用型号帕纳科X’Pert PRO X射线衍射仪(XRD)进行分析,分析选用Cu靶,其中管电压为40 kV,管电流为30 mA。
3. 结果与讨论
3.1. 前驱体的TG-DSC分析
为了研究制备钙钛矿前驱体的质量、温度和分解情况,以LaFe0.5Co0.5O3为例,进行了TG-DSC 测试。图1给出了LaFe0.5Co0.5O3前驱体的TG-DSC曲线。如图可以看出,热重(TG)曲线主要包括四段失重:第一次失重发生在温度110℃之前,这主要是洗涤过程中吸附在前驱体上的水分蒸发所致;第二次失重从150℃开始,一直延续到220℃,且在相应温度范围可观察到DSC曲线上的吸热峰,主要是前驱体中的结晶水脱出导致;第三次失重发生在300℃~500℃,相应温度范围内DSC曲线上出现吸热峰,这主要是前驱体脱出结构水和分解形成相应的氧化物导致;最后一次失重发生在700℃~780℃之间,相应温度范围DSC曲线上呈现吸热峰,归结于部分残余的前驱体分解为金属氧化物和分解产生的金属氧化物相互反应形成钙钛矿的转变过程导致;780℃之后质量不再减少,也没有吸热和放热现象,说明钙钛矿已经形成。综上所述,形成钙钛矿的过程是个吸热过程,可以推知,升高温度有利于钙钛矿的形成。
3.2. 钙钛矿的XRD表征和分析
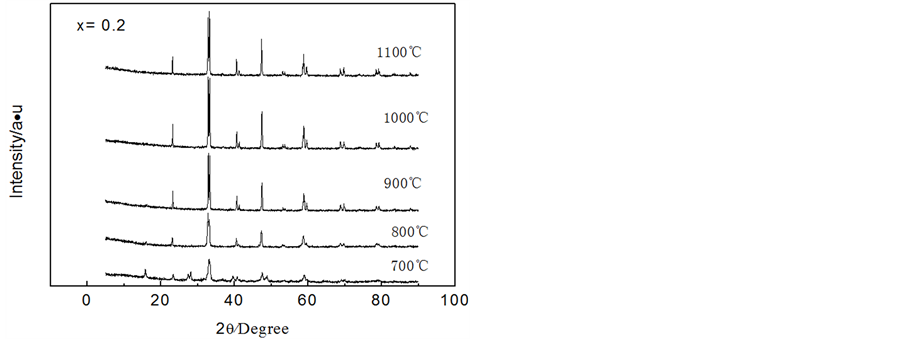
 (x = 0.2、0.5、0.8)前驱体经不同温度煅烧4h后所制备样品的XRD图谱示于图2。
(x = 0.2、0.5、0.8)前驱体经不同温度煅烧4h后所制备样品的XRD图谱示于图2。
由图2(a)可以看出, (x = 0.2)前驱体经过700℃煅烧后,已经有钙钛矿的形成,这与TG-DSC分析结果相一致,但是形成钛矿相的衍射峰强度较低且峰形弥散,而且有其他晶相存在,说明晶体的晶化还不完全,有一定的非晶成分和杂质存在;经过800℃煅烧后,晶相全为钙钛矿;之后,随煅烧温度升高,特征峰分布在23.0˚、32.6˚、33.0˚、47.1˚和58.4˚位置,与LaFe0.25Ni0.75O3(卡片PDF 01-088-0636)的特征峰一致,说明所得产品都形成钙钛矿。同时,从图还可以看出,煅烧温度超过800℃后,随煅烧温度升高,最强特征峰的位置(33.0˚)保持不变,而强度逐渐增强,说明钙钛矿的量随温度的升高而增加;超过1000℃煅烧后,尽管最强特征峰的位置仍保持不变,但是强度又下降,说明经过1000℃煅烧后,钙钛矿部分开始转变和分解。根据谢乐公式(D = 0.89λ/Bcosθ,其中D为样品晶粒尺寸,θ为衍射半角,l为衍射波长,B为半高宽)可得在煅烧温度分别为800℃、900℃和1000℃时,制备出钙钛矿的晶粒尺寸分别约为40 nm、42 nm和64 nm。从而可知,随着煅烧温度的提高,钙钛矿形成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃时获得的钙钛矿量最多,但是与800℃和900℃煅烧后形成的晶粒尺寸相比增加较多。
(x = 0.2)前驱体经过700℃煅烧后,已经有钙钛矿的形成,这与TG-DSC分析结果相一致,但是形成钛矿相的衍射峰强度较低且峰形弥散,而且有其他晶相存在,说明晶体的晶化还不完全,有一定的非晶成分和杂质存在;经过800℃煅烧后,晶相全为钙钛矿;之后,随煅烧温度升高,特征峰分布在23.0˚、32.6˚、33.0˚、47.1˚和58.4˚位置,与LaFe0.25Ni0.75O3(卡片PDF 01-088-0636)的特征峰一致,说明所得产品都形成钙钛矿。同时,从图还可以看出,煅烧温度超过800℃后,随煅烧温度升高,最强特征峰的位置(33.0˚)保持不变,而强度逐渐增强,说明钙钛矿的量随温度的升高而增加;超过1000℃煅烧后,尽管最强特征峰的位置仍保持不变,但是强度又下降,说明经过1000℃煅烧后,钙钛矿部分开始转变和分解。根据谢乐公式(D = 0.89λ/Bcosθ,其中D为样品晶粒尺寸,θ为衍射半角,l为衍射波长,B为半高宽)可得在煅烧温度分别为800℃、900℃和1000℃时,制备出钙钛矿的晶粒尺寸分别约为40 nm、42 nm和64 nm。从而可知,随着煅烧温度的提高,钙钛矿形成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃时获得的钙钛矿量最多,但是与800℃和900℃煅烧后形成的晶粒尺寸相比增加较多。
从图2(b)可以看出,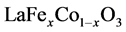 (x = 0.5)前驱体经过800℃煅烧后,已经有钙钛矿的形成,但是钙钛矿特征峰衍射强度较低且峰形弥散,说明晶体的晶化还不完全,有一定的非晶成分;温度超过800℃
(x = 0.5)前驱体经过800℃煅烧后,已经有钙钛矿的形成,但是钙钛矿特征峰衍射强度较低且峰形弥散,说明晶体的晶化还不完全,有一定的非晶成分;温度超过800℃

Figure 1. TG-DSC curves of LaFe0.5Co0.5O3 precursor
图1. LaFe0.5Co0.5O3前驱体的TG-DSC曲线

 (a) (b)
(a) (b)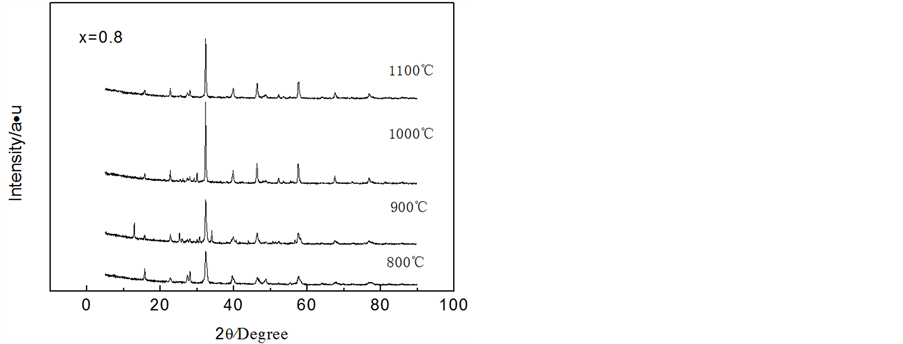 (c)
(c)
Figure 2. XRD patterns of the LaFexCo1-xO3 precursor with x = 0.2, 0.5 and 0.8 calcined at 700˚C - 1100˚C
图2. LaFexCo1-xO3 (x = 0.2、0.5、0.8)前驱体经过700℃~1100℃煅烧后的XRD图谱
煅烧后,其变化趋势与 (x = 0.2)相一致,即煅烧温度为1000℃,特征峰(33.0˚)的强度最高,超过1000℃后特征峰强度又下降。同样根据谢乐公式得出在煅烧温度分别为800℃、900℃和1000℃时,制备出钙钛矿的晶粒尺寸分别约为35 nm、35 nm和46 nm。结果表明,随着煅烧温度的提高,钙钛矿形成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃时获得的钙钛矿量最多,但是与800℃和900℃煅烧后形成的晶粒尺寸相比增加较多。
(x = 0.2)相一致,即煅烧温度为1000℃,特征峰(33.0˚)的强度最高,超过1000℃后特征峰强度又下降。同样根据谢乐公式得出在煅烧温度分别为800℃、900℃和1000℃时,制备出钙钛矿的晶粒尺寸分别约为35 nm、35 nm和46 nm。结果表明,随着煅烧温度的提高,钙钛矿形成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃时获得的钙钛矿量最多,但是与800℃和900℃煅烧后形成的晶粒尺寸相比增加较多。
从图2(c)可以看出, (x = 0.8)前驱体经过800℃煅烧后,已经有钙钛矿的形成,同时存在大量杂质晶体,重要的特征峰强度与
(x = 0.8)前驱体经过800℃煅烧后,已经有钙钛矿的形成,同时存在大量杂质晶体,重要的特征峰强度与 (x = 0.2)和
(x = 0.2)和 (x = 0.5)相比较弱,说明形成的钙钛矿量相对较少;温度超过800℃煅烧后,其特征峰的变化趋势与
(x = 0.5)相比较弱,说明形成的钙钛矿量相对较少;温度超过800℃煅烧后,其特征峰的变化趋势与 (x = 0.2)和
(x = 0.2)和 (x = 0.5)相一致,但有相当量的杂质相存在,说明x = 0.8时,难以获得纯净的
(x = 0.5)相一致,但有相当量的杂质相存在,说明x = 0.8时,难以获得纯净的 钙钛矿相。根据Goldschmdt研究ABO3型钙钛矿中引入的“容限因子(t)”t = (rA + rO)/20.5 (rB + rO)其中,rA、rB、rO分别表示A位离子、B位离子、O位离子的半径)可知,只要满足0.8 ≤ t ≤ 1则有可能形成钙钛矿。由于La3+、Co3+、Fe3+和O2−的半径分别为103.2、74.5、64.5和140 pm,可知当B位为Fe3+和Co3+时,容限因子分别为0.84和0.80,满足离子半径形成钙钛矿的必要条件。但是,由于Fe和Co的晶体构型分别为立方和六方,不能满足其形成无限置换固溶体的条件,从而导致x = 0.8时,超过了固溶体的极限,使得形成的钙钛矿不纯净。在煅烧温度分别为900℃、1000℃和1100℃时,根据谢乐公式得出钙钛矿的晶粒尺寸分别约为30 nm、32 nm和46 nm。
钙钛矿相。根据Goldschmdt研究ABO3型钙钛矿中引入的“容限因子(t)”t = (rA + rO)/20.5 (rB + rO)其中,rA、rB、rO分别表示A位离子、B位离子、O位离子的半径)可知,只要满足0.8 ≤ t ≤ 1则有可能形成钙钛矿。由于La3+、Co3+、Fe3+和O2−的半径分别为103.2、74.5、64.5和140 pm,可知当B位为Fe3+和Co3+时,容限因子分别为0.84和0.80,满足离子半径形成钙钛矿的必要条件。但是,由于Fe和Co的晶体构型分别为立方和六方,不能满足其形成无限置换固溶体的条件,从而导致x = 0.8时,超过了固溶体的极限,使得形成的钙钛矿不纯净。在煅烧温度分别为900℃、1000℃和1100℃时,根据谢乐公式得出钙钛矿的晶粒尺寸分别约为30 nm、32 nm和46 nm。
上述结果表明,当x = 0.2和0.5时, 钙钛矿前驱体经过700℃和800℃煅烧后已经形成钙钛矿,之后随煅烧温度提高,钙钛矿生成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃获得的钙钛矿量最多,而当x = 0.8时,煅烧得到钙钛矿含有杂质;随着Fe取代Co的量增加,形成钙钛矿尺寸晶粒尺寸逐渐降低,这可能与Fe3+半径小于Co3+半径有关。
钙钛矿前驱体经过700℃和800℃煅烧后已经形成钙钛矿,之后随煅烧温度提高,钙钛矿生成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃获得的钙钛矿量最多,而当x = 0.8时,煅烧得到钙钛矿含有杂质;随着Fe取代Co的量增加,形成钙钛矿尺寸晶粒尺寸逐渐降低,这可能与Fe3+半径小于Co3+半径有关。
4. 结论
采用沉淀法可以制备出 钙钛矿,合成钙钛矿过程有四段失重,分别在110℃之前、150℃~220℃、300℃~500℃和700℃~780℃。当x = 0.2和0.5时,LaFexCo1−xO3钙钛矿前驱体经过700℃和800℃煅烧后已经开始形成钙钛矿,之后随煅烧温度提高,钙钛矿生成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃获得的钙钛矿量最多,此时晶粒尺寸为64 nm (x = 0.2)和46 nm (x = 0.5),但是与800℃和900℃煅烧后形成的晶粒尺寸相比增加较多。当x = 0.8时,LaFexCo1−xO3钙钛矿前驱体经过煅烧得到钙钛矿含有杂质,这与Fe和Co的晶体构型分别为立方和六方,不能满足其形成无限置换固溶体的条件有关。
钙钛矿,合成钙钛矿过程有四段失重,分别在110℃之前、150℃~220℃、300℃~500℃和700℃~780℃。当x = 0.2和0.5时,LaFexCo1−xO3钙钛矿前驱体经过700℃和800℃煅烧后已经开始形成钙钛矿,之后随煅烧温度提高,钙钛矿生成量逐渐增加,晶粒尺寸也缓慢增加,煅烧温度为1000℃获得的钙钛矿量最多,此时晶粒尺寸为64 nm (x = 0.2)和46 nm (x = 0.5),但是与800℃和900℃煅烧后形成的晶粒尺寸相比增加较多。当x = 0.8时,LaFexCo1−xO3钙钛矿前驱体经过煅烧得到钙钛矿含有杂质,这与Fe和Co的晶体构型分别为立方和六方,不能满足其形成无限置换固溶体的条件有关。
致谢
感谢试验中、论文写作过程中给予分析指导和理论分析的石风俊教授,是您无限的耐心和渊博的知识促成试验的顺利完成。