1. 引言
随着社会经济的飞速发展,环境污染成为当前人们面临的重大问题。在生产和应用的工业、农业和生活废水中包含多种有机和无机物,其中有机物是最主要的一类,由于其成分相对复杂、色度高、毒性大,并且难以被生物降解,已成为污水处理的难点和水体的重要污染源之一 [1] [2] ;而无机污染物最常见的包括金属离子与无机阴离子,目前金属离子污染的处理方法包括高分子络合、活性炭吸附、沉淀法、离子交换及电化学法等 [3] [4] 。近年来,半导体光催化由于可以直接利用太阳能、高效、无污染等优点,已成为环境净化领域的研究热点 [5] 。光催化为有机物与金属离子污染物的消除提供了一条新途径 [6] - [11] ,比如Serpone [12] 等报道了用TiO2光催化法从Au(CN)3−中还原Au,同时氧化CN−为NH3和CO2,并指出将该方法用于电镀工业废水的处理,不仅能还原镀液中的贵金属,而且还能消除镀液中氰化物对环境的污染,是一种具有实用价值的处理方法。
镉(Cd2+)是污水中广泛分布的有害重金属,对人体和环境有很大的危害性,因而开发高效、廉价的治理镉污染的方法和材料显得格外重要。最近,我们提出了镉离子固化的策略,即向镉离子溶液中添加磷酸盐,得到Cd5H2(PO4)4∙4H2O花状微球。该物质经过煅烧处理后转变为Cd5(PO4)2P2O7微球,后者在紫外光照射下可以高效降解染料废水 [13] 。
在本文中,我们在前期工作基础上提出了一步光催化同时净化Cd2+和染料溶液(罗丹明B,RhB)的思路,即在固化镉离子的同时将水溶液中的有机染料一并降解,该法更加简单、有效。在实验中重点研究了镉盐、磷酸盐、有无光照对镉离子固化和光催化降解RhB性能的影响。
2. 实验部分
2.1. 实验试剂
乙酸镉(Cd(CH3COO)2),硝酸镉(Cd(NO3)2),罗丹明B(RhB),分析纯,购于国药集团化学试剂有限公司;硫酸镉(CdSO4),磷酸钠(Na3PO4),磷酸氢二钠(Na2HPO4),磷酸二氢钠(NaH2PO4),分析纯,购于天津市广成化学试剂有限公司。
2.2. 实验过程
将0.9991 g Cd(CH3COO)2溶于100 mL去离子水中搅拌10 min至完全溶解,加入50 mL罗丹明B溶液(10 mg/L),磁力搅拌10 min使之混合均匀得溶液A。称取0.9 g Na2HPO4溶于60 mL去离子水中得溶液B,将B溶液逐滴加入到A溶液中,得到白色沉淀。滴加完成后,将悬浮液在暗室环境中持续搅拌30 min以建立催化剂与有机染料之间的吸附–脱附平衡。以4个波长为254 nm的紫外灯管为辐射光源进行光催化降解实验,每隔一定时间抽取4 mL溶液离心得清液,在554 nm处检测其吸光度,降解率表示为C/C0,其中C0是罗丹明B的初始吸光度值,C表示在一定时间光照后的吸光度值。
2.3. 样品表征
本实验采用日本理学社Miniflex 600型X射线衍射仪(XRD, Cu Kα, λ = 0.154 nm)表征产物物相;采用JEOL JSM-6700型场发射扫描电子显微镜(SEM)表征产物的表面形貌;采用UV-756CRT型紫外分光光度计测定染料的吸光度值。
3. 结果与讨论
3.1. 一步光催化净化机制
本文提出的一步光催化净化Cd2+和RhB分子的过程如图1所示。首先Cd2+与PO43−在常温下发生沉淀反应生成Cd5H2(PO4)4∙4H2O (反应1);在紫外光照射下,Cd5H2(PO4)4∙4H2O产生光生电子(e−)和空穴(h+) (反应2),空穴(h+)和电子(e−)会与溶液中的水或氧气反应生成活性自由基(羟基自由基(∙OH)或超氧自由基(∙O2−)) (反应3和4);在自由基的作用下,RhB分子被分解成小分子产物(反应5)。
Cd2+固化反应:
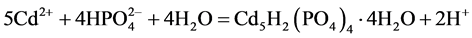 (1)
(1)
在紫外光激发下RhB的光催化降解过程:

Figure 1. The one-step photocatalytic purification route for solutions containing Cd2+ and RhB
图1. 一步光催化净化Cd2+和RhB过程图
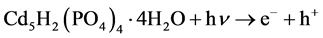 (2)
(2)
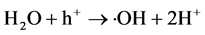 (3)
(3)
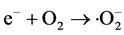 (4)
(4)
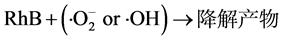 (5)
(5)
采用分光光度计测定了在紫外光照射下RhB的分解情况,如图2所示,随光照时间的增加,RhB在554 nm处的特征吸收峰峰值逐渐降低,10 min后完全消失,溶液的颜色也由最初的粉红色变成无色,这说明RhB的结构被破坏并被分解成小分子结构。
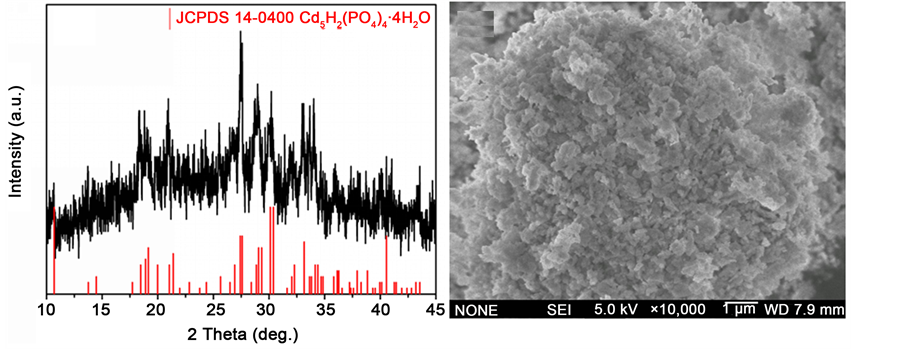
待RhB溶液完全脱色后,将溶液中的沉淀洗涤、干燥,对其晶相结构和形貌进行表征。XRD结果如图3(a)所示,所得产物在2θ为10.689˚、18.469˚、19.153˚、26.914˚、27.593˚、30.126˚和30.399˚出现了特征衍射峰,与JCPDS标准卡14-0400一致,说明所得产物为Cd5H2(PO4)4∙4H2O。图3(b)为产物的SEM图,从图中可以看出,Cd5H2(PO4)4∙4H2O是由纳米颗粒团聚形成的无规则结构,纳米颗粒尺度在几百纳米左右。

Figure 2. The spectral changes of RhB as a function of irradiation time under UV light
图2. RhB随光照时间的光谱变化图
 (a) (b)
(a) (b)
Figure 3. XRD and SEM images of Cd5H2(PO4)4∙4H2O
图3. Cd5H2(PO4)4∙4H2O的XRD图谱和SEM图
3.2. 影响因素研究
从上述表征结果可以看出,该一步法不仅可以将有机污染物RhB降解,还能将Cd2+成功固化得到紫外型光催化剂Cd5H2(PO4)4∙4H2O。接下来我们又考察了不同镉盐种类、不同磷酸盐种类和有无光照对所得固体产物结构和RhB降解活性的影响。
3.2.1. 不同镉盐的影响
首先研究了Cd(CH3COO)2,Cd(NO3)2和CdSO4三种镉盐与Na2HPO4反应后产物的结构和对RhB降解活性的影响。固化不同镉盐得到的产物XRD (图4(a))结果显示,三种镉盐均可被固化,所得产物均为Cd5H2(PO4)4∙4H2O,相比之下,由Cd(NO3)2得到的样品衍射峰强度高于CdSO4和Cd(CH3COO)2制备的样品,说明前者的结晶性更好。从图4(b)可以看出,三种镉盐固化后光催化降解RhB的活性顺序为Cd(NO3)2 ﹥ CdSO4 ﹥ Cd(CH3COO)2,这可能与所得产物的结晶性有关。文献报道,光催化材料的结晶度越高,表面及体相的缺陷或杂质越少,越有利于光生电子的高效传递及分离 [14] 。此外,体系中存在的阴离子对光催化反应也有重要影响,如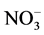 在紫外光照射下会生成NO2自由基,具有极强氧化性,也能够促进RhB的分解 [15] 。
在紫外光照射下会生成NO2自由基,具有极强氧化性,也能够促进RhB的分解 [15] 。
3.2.2. 不同磷酸盐的影响
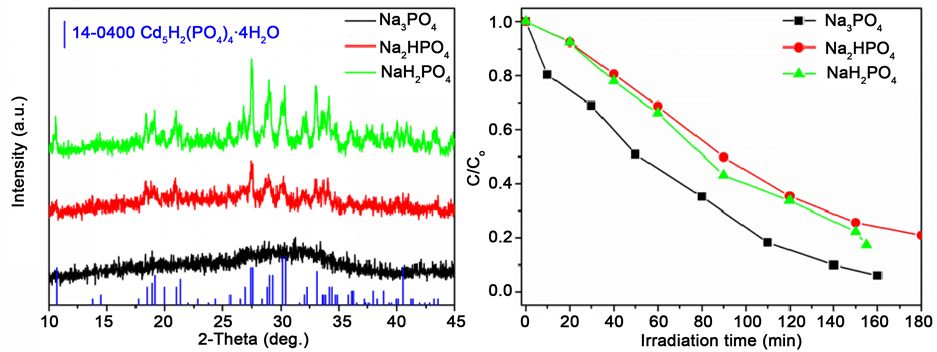
实验中还探究了Na3PO4、Na2HPO4和NaH2PO4三种磷酸盐固化Cd(CH3COO)2反应后所得产物的晶相结构及其对RhB降解活性的影响。从图5(a)可以看出,由Na2HPO4和NaH2PO4固化得到的产物均为Cd5H2(PO4)4∙4H2O;而由Na3PO4固化得到的产物无明显衍射峰。我们前期工作已证明pH在Cd2+固化过程中发挥重要作用,高pH条件下得到的产物为无定型相材料 [13] 。尽管Na3PO4固化得到的产物结晶性差,但产物的颗粒尺寸更小、表面活性位更多,在降解RhB时表现出更高的活性(图5(b))。
3.2.3. 光照的影响
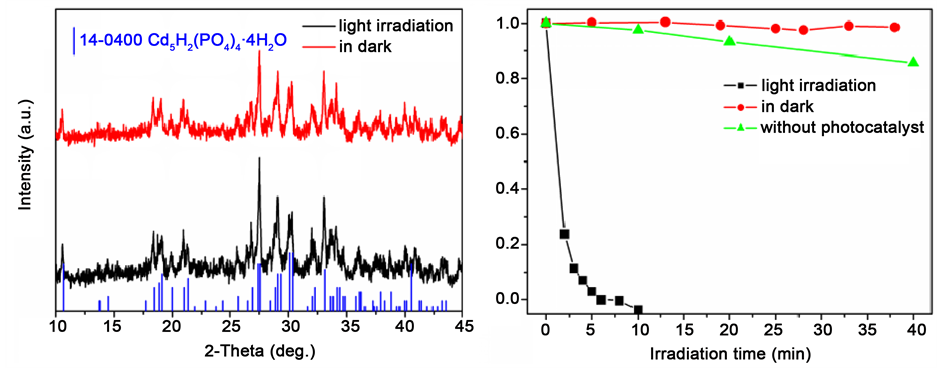
考察了光照对Cd2+固化和RhB降解的影响。在保证其它条件一致的情况下,一组实验用紫外灯照射,另一组实验不用紫外灯照射。图6(a)的XRD图显示,有无紫外灯照射,Cd2+都可以被固化为Cd5H2(PO4)4∙4H2O。但在没有光照时,RhB分子不会被分解(图6(b));另外在紫外光照而不加催化剂时,RhB分子也不会被降解。这说明光照和Cd5H2(PO4)4∙4H2O催化剂对RhB的降解是必要条件。
 (a) (b)
(a) (b)
Figure 4. (a) XRD images of the samples derived from different Cd2+ and (b) the photocatalytic curves for RhB degradation
图4. (a) 固化不同镉盐所得产物的XRD图;(b) RhB溶液的光催化降解图
 (a) (b)
(a) (b)
Figure 5. (a) XRD images of the samples derived from different 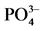 and (b) the photocatalytic curves for RhB degradation
and (b) the photocatalytic curves for RhB degradation
图5. (a) 不同磷酸盐固化产物的XRD图;(b) RhB溶液的光催化降解图
 (a) (b)
(a) (b)
Figure 6. (a) XRD images of the samples derived from light irradiation or in dark; (b) the curves for RhB degradation
图6. (a) 有无光照下所得产物的XRD图;(b) RhB溶液的催化降解图
4. 结论
采用原位固化的方法成功固化镉离子,同时利用所得固体产物的光催化性质将有机染料RhB降解去除,实现了一步净化Cd2+和RhB溶液的目的。实验中还探究了不同镉盐、磷酸盐、光照对固化产物的结构及光催化降解RhB活性的影响。该研究提出了一步净化含有重金属离子和有机污染物分子溶液的简单方法,在实际废水处理过程中具有一定的潜在实施价值,但考虑到实际废水中成分的复杂性,相关研究仍然具有挑战性和研究价值。
致谢
感谢国家自然科学基金(21103193)、国家大学生创新训练项目(201510446061)和曲阜师范大学实验室开放基金(sk201403)对本文的资助。
NOTES
*通讯作者。