1. 引言
硝化作用是指在硝化菌作用下,把氨氮氧化成硝氮的过程。随着工农业的高速发展,人类活动对河口近岸环境的影响越来越突出,特别是最近50年来,人类活动已使河流输送的溶解无机氮增加了2~4倍 [1] 。河口中氮负荷的大量增加已经引起多种环境效应,赤潮频发和河口缺氧就是典型的例子,已发展成为当今世界面临的一个全球性重大环境问题。
硝化作用可以将水体和沉积物中的氨氮氧化为 ,使氮素发生形态转化,从而降低氨氮的毒性 [2] ,而产生的
,使氮素发生形态转化,从而降低氨氮的毒性 [2] ,而产生的 通过反硝化作用形成N2和N2O,使氮返回到大气中,从而完成一次氮的生物地球化学循环。硝化与反硝化作用是河口近岸氮素营养盐去除的重要途径,对于缓解水体的富营养化状况具有重要作用。硝化作用是耗氧和产生质子的过程,在氮污染严重的河口、近岸水域,硝化作用是造成水体缺氧,特别是底层水体和沉积物缺氧的重要原因 [3] [4] [5] ;此外沉积物和水界面强烈的硝化作用,会明显降低沉积物间隙水的pH值,从而导致沉积物中重金属离子的溶出,增加重金属的环境毒性 [6] 。硝化作用还是大气中N2O的重要来源 [7] [8] ,N2O是第二大温室气体,也是破坏大气臭氧层的主要化合物之一。全球N2O中有20%来源于海洋,其中的60%以上来自近岸。河口、近岸水域的硝化作用的强度必然会影响到大气N2O的分布,进而影响全球的气候系统。因此,研究河口、近岸水域的硝化过程不仅有助于了解河口、近岸水体对氮的自净能力,探明河口、近海缺氧区的形成机制,而且对研究全球气候变化也有重要意义。
通过反硝化作用形成N2和N2O,使氮返回到大气中,从而完成一次氮的生物地球化学循环。硝化与反硝化作用是河口近岸氮素营养盐去除的重要途径,对于缓解水体的富营养化状况具有重要作用。硝化作用是耗氧和产生质子的过程,在氮污染严重的河口、近岸水域,硝化作用是造成水体缺氧,特别是底层水体和沉积物缺氧的重要原因 [3] [4] [5] ;此外沉积物和水界面强烈的硝化作用,会明显降低沉积物间隙水的pH值,从而导致沉积物中重金属离子的溶出,增加重金属的环境毒性 [6] 。硝化作用还是大气中N2O的重要来源 [7] [8] ,N2O是第二大温室气体,也是破坏大气臭氧层的主要化合物之一。全球N2O中有20%来源于海洋,其中的60%以上来自近岸。河口、近岸水域的硝化作用的强度必然会影响到大气N2O的分布,进而影响全球的气候系统。因此,研究河口、近岸水域的硝化过程不仅有助于了解河口、近岸水体对氮的自净能力,探明河口、近海缺氧区的形成机制,而且对研究全球气候变化也有重要意义。
河口、近岸水域硝化作用受复杂的物理、化学、生物等因子共同控制,且地区性差异很大。目前,国内外对河口、近岸水域的硝化作用的研究主要集中在河口沉积物和滨海湿地沉积物 [2] [9] [10] [11] ,对水体的硝化作用的研究还比较缺乏,对硝化作用发生的条件,以及环境因素对硝化作用产生影响的机制尚不清楚。本文以受人类影响比较严重的杏林湾为研究区域,通过现场调查结合实验室模拟培养,研究了杏林湾表层水体溶解无机氮和硝化作用强度的空间分布和季节变化,探讨了温度、pH、氨氮浓度以及颗粒物等环境因素对硝化作用的影响,以深化对河口、近岸水域氮循环机制的认识。
2. 材料与方法
2.1. 研究区域概况
杏林湾位于厦门市北部的集美区内,杏林湾水域由后溪、港头、杏林等区域的小溪流或沟渠汇集而成,原属亚热带海湾滩涂。经1956年人工修筑集杏海堤后,形成一个封闭的淡水水库。杏林湾水库流域面积142 km2,集水面积67.3 km2,库容面积约2.2 km2,平均水深约2.5 m,最大深度5.5 m [12] 。水库北部形成淡水水域,南部由于厦门西海域海水通过集杏海堤渗透进入,形成咸淡混合水体。杏林湾外海域潮汐为正规半日潮,由于集杏海堤和集美水闸等作用,把杏林湾水体与外海水体基本隔断,使其受外海潮汐作用较小。随着厦门城镇化的快速推进,工业、农业、水产养殖、畜禽养殖及生活污水等不断排入水库,使库区氮磷浓度严重超标达到劣五类水,水体富营养化日益严重 [13] 。
2.2. 站位设置及样品采集
于2011年8月(夏季)和2012年2月(冬季)沿杏林湾水库的上游到下游分别设定5个站位(见图1),采集表层水样进行氮素营养盐的形态分析,并用多功能水质分析仪(德国WTW,Multi 3420)现场测定表层温度、盐度、溶解氧、pH。同时在水库的上游S1站、中游S2站和下游S5分别进行硝化作用实验室模拟培养。为了研究环境因素对硝化作用的影响,于2012年3月期间取S5站位的表层水样进行实验室受控培养。各个站位的表层水样用小桶采集,装入20 L的聚碳酸酯瓶中,马上运回实验室进行相关指标的测定和培养实验,样品采集和后续处理的时间间隔一般不超过3 h。所有采样器械、容器、滤膜、培养装置均预先经过1.0 mol∙L−1的HCl酸泡24 h,再用超纯水洗至中性后使用。
2.3. 硝化速率的测定
硝化速率的测定采用抑制剂法 [4] 。一般认为自然环境中的硝化作用分两步完成,第一步是氨在氨氧化菌(AOB)的作用下被氧化为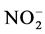 ;第二步是
;第二步是 在亚硝酸盐氧化菌(NOB)的作用下被氧化为
在亚硝酸盐氧化菌(NOB)的作用下被氧化为 。硝化作用可简单表示如下:
。硝化作用可简单表示如下:
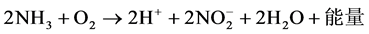 (1)
(1)
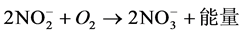 (2)
(2)
抑制剂法的测定原理如下:在培养过程中加入NaClO3抑制剂,可以阻止硝化反应的第二步反应,即 被氧化成
被氧化成 ,但不会阻止NH3氧化成
,但不会阻止NH3氧化成 的第一步反应,因此随着培养的进行,
的第一步反应,因此随着培养的进行, 浓度将逐渐升高,测量
浓度将逐渐升高,测量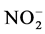 浓度的升高速率,就可以得到氨氧化速率。相应地在培养过程中加入烯丙基硫尿(ATU)抑制剂,可以阻止硝化反应的第一步反应,但不会阻止第二步反应,因此
浓度的升高速率,就可以得到氨氧化速率。相应地在培养过程中加入烯丙基硫尿(ATU)抑制剂,可以阻止硝化反应的第一步反应,但不会阻止第二步反应,因此 浓度将逐渐降低,测量
浓度将逐渐降低,测量 浓度的降低速率,就可以得到
浓度的降低速率,就可以得到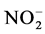 氧化速率。本文报道的氨氧化速率和
氧化速率。本文报道的氨氧化速率和 氧化速率均为培养48 h的平均速率。
氧化速率均为培养48 h的平均速率。
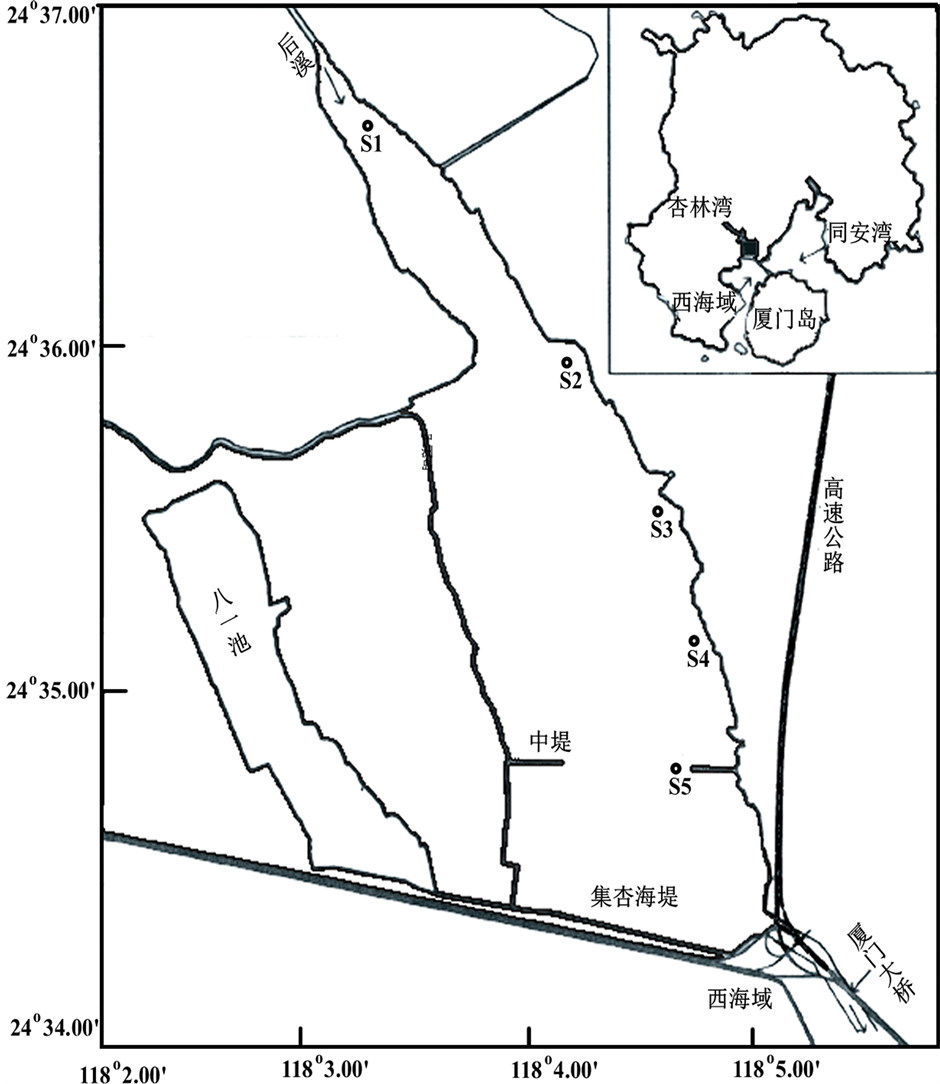
Figure 1. Location of sampling sites in Xinglin Bay
图1. 杏林湾水域的调查站位
测定方法简述如下:将上述水样分装入3个洗净和灭菌后的4 L的棕色玻璃瓶子中,装入之前先用水样润洗三遍。其中1号瓶中加100 mg∙L−1 ATU,2号瓶中加10 mg∙L−1氯酸钠,3号瓶中加100 mg∙L−1 ATU和氯酸钠10 mg∙L−1作为控制样。置于恒温培养箱避光培养,培养温度控制与现场采样时的水温相当,即夏季为30℃,冬季为15℃,培养周期48 h。分别在培养之初(0 h)、24 h、48 h取培养水样,经0.45 μm纤维滤膜过滤后,用于测定亚硝酸盐氮的浓度。
2.4. 环境因素对硝化作用强度的影响
采用单因素实验法研究环境因素对硝化作用的影响,即在实验中,只有一个因素在变化,其余的因素保持不变。即采集S5站位的表层水样,分别在不同的温度下进行培养以研究温度的影响;同样采集S5站位的表层水,用稀盐酸或稀氢氧化钠调节成不同的pH,然后在30℃下进行培养以研究pH的影响;取S5站位的表层水样,加入(NH4)2SO4调整成含不同的 浓度,然后在30℃下进行培养以研究
浓度,然后在30℃下进行培养以研究 浓度的影响;颗粒物的影响则采用过3 μm滤膜的水样和未过滤的水样在30℃进行对照培养。
浓度的影响;颗粒物的影响则采用过3 μm滤膜的水样和未过滤的水样在30℃进行对照培养。
2.5. 溶解无机氮浓度测定
溶解无机氮包括氨氮、亚硝氮和硝氨。样品采集后马上运回实验室用0.45 μm的纤维素滤膜过滤,滤液用于测定氨氮、亚硝氮和硝氨的浓度。其中氨氮浓度测定采用靛酚蓝法 [4] , -N浓度测定采用盐酸萘乙二胺法 [4] ,
-N浓度测定采用盐酸萘乙二胺法 [4] ,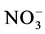 -N浓度测定采用锌镉还原法 [14] 。
-N浓度测定采用锌镉还原法 [14] 。
3. 结果与分析
3.1. 杏林湾水体溶解无机氮的空间分布和季节变化
考虑到杏林湾水库水体较浅,平均深度仅2.5 m,因此只对表层水体进行采样和观测。如表1所示,2011年8月(夏季)杏林湾表层水体的盐度范围在0.3~0.9之间,下游因受海水渗入的影响盐度略高于上游,但差别不大;水温在29.4℃~30.0℃之间,空间差异不明显;溶解氧(DO)浓度范围为4.8~6.5 mg∙L−1,均底于现场温度、盐度和气压条件下水体DO的饱和度,存在不同程度的氧亏损现象,这主要是因为水体存在强烈的生物耗氧,夏季水体总耗氧速率可高达2.1~3.5 mg∙L−1∙d−1 (何碧烟等,未发表数据)。2012年2月(冬季)因为上游来水较夏季少,表层水体盐度略高于夏季,范围在0.3~1.2之间;水温明显低于夏季,杏林湾水浅,水温基本受气温变化控制,空间差异很小;溶解氧浓度范围为6.0~9.2 mg∙L−1,比夏季高,主要是因为冬季气温低,氧在水中的溶解度较大,此外还因为水温较低,微生物耗氧速率也比较低。
夏季杏林湾水库 -N的浓度范围为30~96 μmol∙N∙L−1,
-N的浓度范围为30~96 μmol∙N∙L−1,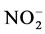 -N的浓度约为25~33 μmol∙N∙L−1,
-N的浓度约为25~33 μmol∙N∙L−1, -N的浓度约是230~281 μmol∙N∙L−1。冬季
-N的浓度约是230~281 μmol∙N∙L−1。冬季 -N的浓度范围为13~93 μmol∙N∙L−1,
-N的浓度范围为13~93 μmol∙N∙L−1, -N的浓度约为21~24 μmol∙N∙L−1,
-N的浓度约为21~24 μmol∙N∙L−1, -N的浓度约166~287 μmol∙N∙L−1。三态氮中以
-N的浓度约166~287 μmol∙N∙L−1。三态氮中以 -N为主,夏季
-N为主,夏季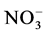 -N平均占总溶解无机氮(TDIN)的74%,冬季占78%,其次是
-N平均占总溶解无机氮(TDIN)的74%,冬季占78%,其次是 -N,夏季和冬季
-N,夏季和冬季 -N分别约占TDIN的17%和14%,
-N分别约占TDIN的17%和14%,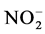 -N的含量最少,在两个季节均约占TDIN的8%。
-N的含量最少,在两个季节均约占TDIN的8%。
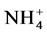 -N的空间差异最显著,无论是春季或者夏季最高值都出现在水库上端的S1站,可能是由于上游受杏林居民排污影响较大,而且上游水浅,沉积物再悬浮可能也是造成水体
-N的空间差异最显著,无论是春季或者夏季最高值都出现在水库上端的S1站,可能是由于上游受杏林居民排污影响较大,而且上游水浅,沉积物再悬浮可能也是造成水体 -N高的原因;水库中游区域由于没有污水输入的影响,
-N高的原因;水库中游区域由于没有污水输入的影响, -N和
-N和 -N浓度比较低;靠下游的S4站附近有2个排污口,受污水输入的影响营养盐浓度也比较高。从季节变化看,夏季的
-N浓度比较低;靠下游的S4站附近有2个排污口,受污水输入的影响营养盐浓度也比较高。从季节变化看,夏季的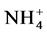 -N和
-N和 -N浓度均大于冬季,可能是因为夏季暴雨频发,雨水把杏林湾周边富含营养盐的污水冲刷进入杏林湾水库造成的。这与文献报告杏林湾水库丰水期的污染指数比枯水期高是一致的 [12] 。
-N浓度均大于冬季,可能是因为夏季暴雨频发,雨水把杏林湾周边富含营养盐的污水冲刷进入杏林湾水库造成的。这与文献报告杏林湾水库丰水期的污染指数比枯水期高是一致的 [12] 。
3.2. 杏林湾水体的硝化速率
杏林湾表层水体的氨氧化速率和 氧化速率的分布如表2所示,夏季水体氨氧化速率在5.28~9.33 μmol∙N∙L−1∙d−1之间,平均值为7. 15 ± 2.04 μmol∙N∙L−1∙d−1,亚硝酸盐氧化速率在2.64~8.78 μmol∙N∙L−1∙d−1之间,平均值为5.42 ± 3.10 μmol∙N∙L−1∙d−1。冬季水体氨氧化速率在1.16~4.52 μmol∙N∙L−1∙d−1之间,平均值为2. 93 ± 1.69 μmol∙N∙L−1∙d−1,亚硝酸盐的氧化速率在0.95~1.92 μmol∙N∙L−1∙d−1之间,平均值为1.55 ± 0.52 μmol∙N∙L−1∙d−1。从总体上看,水体的氨氧化速率大于亚硝酸盐的氧化速率,这可能是导致水体亚硝
氧化速率的分布如表2所示,夏季水体氨氧化速率在5.28~9.33 μmol∙N∙L−1∙d−1之间,平均值为7. 15 ± 2.04 μmol∙N∙L−1∙d−1,亚硝酸盐氧化速率在2.64~8.78 μmol∙N∙L−1∙d−1之间,平均值为5.42 ± 3.10 μmol∙N∙L−1∙d−1。冬季水体氨氧化速率在1.16~4.52 μmol∙N∙L−1∙d−1之间,平均值为2. 93 ± 1.69 μmol∙N∙L−1∙d−1,亚硝酸盐的氧化速率在0.95~1.92 μmol∙N∙L−1∙d−1之间,平均值为1.55 ± 0.52 μmol∙N∙L−1∙d−1。从总体上看,水体的氨氧化速率大于亚硝酸盐的氧化速率,这可能是导致水体亚硝

Table 1. Spatial and seasonal variation of physical and chemical parameters and inorganic nitrogen in surface water of the Xinglin Bay
表1. 杏林湾表层水体的理化参数和无机氮的空间分布与季节变化
注:DO指溶解氧;TDIN指总溶解无机氮,包括氨氮、亚硝氮和硝氮。

Table 2. Spatial and seasonal variation of nitrification rates in surface water of the Xinglin Bay
表2. 杏林湾表层水体硝化速率的空间分布和季节变化
注:速率单位均为μmol∙N∙L−1∙d-1。
酸盐积累的主要原因。从空间分布看,水库上游S1站和下游的S5站的氨氧化速率和亚硝酸盐的氧化速率均高于中游的S2站,氨氧化速率的空间分布和 -N的空间分布基本一致,说明氨氧化速率的空间差异可能主要受
-N的空间分布基本一致,说明氨氧化速率的空间差异可能主要受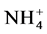 -N浓度的空间差异决定。从季节变化看,夏季氨氧化速率和亚硝酸盐的氧化速率均高于冬季,其原因可能是夏季水体的平均温度大于冬季,促进了硝化作用,同时夏季水体的溶解无机氮的浓度也比冬季高,为硝化细菌提供充足的反应底物,有利于硝化作用的进行。
-N浓度的空间差异决定。从季节变化看,夏季氨氧化速率和亚硝酸盐的氧化速率均高于冬季,其原因可能是夏季水体的平均温度大于冬季,促进了硝化作用,同时夏季水体的溶解无机氮的浓度也比冬季高,为硝化细菌提供充足的反应底物,有利于硝化作用的进行。
4. 讨论
4.1. 温度对氨氧化速率的影响
如表2所示,杏林湾水体的硝化作用强度存在显著的空间差异和季节变化,推测可能与无机氮的浓度、水体的温度、pH等环境因素有关。为了确认环境因素对硝化作用的影响,本研究于2012年3月期间采集S5站位水样进行实验室受控培养。实验结果如图2所示。
4.1.1. 温度对氨氧化速率的影响
从图2a可以看出,在温度5℃~30℃之间,随着温度的升高,硝化速率显著提高。本研究结果显示硝化作用最适宜的温度是30℃左右,温度太低会抑制硝化作用,当温度为5℃时硝化作用几乎完全被抑制,氨氧化速率只有0.22 μmol∙N∙L−1∙d−1,但是温度过高也会抑制硝化作用,例如温度35℃时氨氧化速率反而有所降低,硝化速率随温度的这种变化趋势可能是微生物适应环境的结果。根据我们多年的观测,杏林湾水体的温度在全年的大部分时间都处于20℃~30℃之间,这也是该区域微生物比较适应的生长温度。在此温度范围内,温度升高10℃氨氧化速率提高2.9倍(即Q10 = 2.9)。Jones等 [15] 通过培养测得海洋和淡水硝化细菌的典型Q10值范围为1~3.5之间,与本文的测定结果吻合。温度对硝化作用的影响很好地解释了杏林湾水体夏季硝化作用比冬季强的现象。
4.1.2. pH对硝化作用的影响
pH从6.5提高到8.0,水体的硝化作用强度有一定程度的提高。当pH为8.0时,氨氧化速率达到最大,为11.78 μmol∙L−1∙d−1,是pH为6.5时1.36倍。然而,pH进一步升高到9.0,氨氧化速率反而有所下降,说明弱碱性条件下有利于硝化作用,而弱酸性和较强的碱性条件下会对硝化作用产生抑制。pH对硝化作用有重要的影响,一方面pH可以影响氨的解离平衡,从而影响氨氮中的游离态氨(NH3)的比例。我们知道氨氮包括离子态的 和游离态的NH3,二者存在如下平衡:
和游离态的NH3,二者存在如下平衡:

Figure 2. The influence of temperature, pH, ammonia concentration and particulate matter on ammonia oxidation rates (Ra)
图2. 温度、pH、氨的浓度和颗粒物对氨氧化速率(Ra)的影响
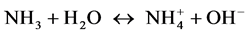 (3)
(3)
当水体pH发生变化时,上述平衡也随之移动,由上述解离平衡(3)可以推导出游离氨NH3浓度的计算公式(4):
 (4)
(4)
式中:TAN代表总氨氮浓度(mmol∙L−1);Kb是氨的解离常数;Kw是水的离子积常数。Kb和Kw都是温度的函数,Kb/Kw随温度变化可以由公式(5)近似计算 [16] :
 (5)
(5)
式中:t代表温度(℃)。由(4)和(5)可以计算出,在温度为30℃时,pH从6.5升高到8.0,游离NH3占TAN的比例从~0.3%升高到~7.7%,而NH3被认为是AOB作用真正底物,因此适度升高pH值可以促进氨氧化作用。另一方面,当pH过高时会影响氨氧化酶的活性,从而降低氨氧化速率,这在其它水域也有类似的报道 [17] 。
4.1.3. 氨浓度对硝化作用的影响
在低浓度下随着水样中氨氮浓度的上升,硝化作用强度逐渐上升,氨的浓度从1.0 mg∙N∙L−1升高到3.0 mg∙N∙L−1时,氨氧化速率增加了~55%。氨氮是AOB作用的底物,适度增加底物的浓度能够促进AOB的生长,从而提高氨氧化速率。但是氨浓度进一步升高到6.0 mg∙N∙L−1氨氧化速率并没有进一步升高,反而略有下降,表现为弱抑制现象。而此时水体中游离氨的浓度~0.45 mg∙N∙L−1,和上述pH等于9.0时水体中游离氨的浓度~0.46 mg∙N∙L−1相当,预示着较高氨浓度和较高pH对硝化作用的抑制机理可能是相似的。值得注意的是以往的研究表明,AOB对氨氮的耐受性比较强,游离NH3的浓度大于10~150 mg∙N∙L−1才会对AOB产生抑制作用 [18] 。本实验设定的氨氮浓度范围1.0~8.0 mg∙N∙L−1,对应的游离态NH3浓度为0.077~0.62 mg∙N∙L−1,远低于前人报道的NH3对AOB的抑制阈值 [18] 。本研究涉及的是天然水体中的氨氧化菌,因其长期生长在氨浓度较低(0.3~1.3 mg∙N∙L−1,见表1)的环境中,不能适应短期内氨浓度的大幅升高是可以理解的。类似的现象在其它天然水体中也有报道,例如,Isnansetyo等 [17] 报告氨氮浓度大于200 μmol∙N∙L−1 (即2.8 mg∙N∙L−1)时,会抑制Ariake海水体的硝化作用,这进一步说明了微生物对环境的适应性。
4.1.4. 颗粒物对硝化作用的影响
图2d所示,经过3 μm滤膜过滤后的水样培养测得的氨氧化速率大约只有未过滤水样的1/5,说明颗粒物的存在可以显著提高氨氧化作用。这是因为一方面经过过滤后,水中AOB和NOB的数量减少,从而使硝化作用减弱 [19] 。另一方面,颗粒物是一种吸附载体,可以通过吸附水体中的氨氮并在颗粒物表面提供细菌氧化的场所,从而加快硝化反应 [19] 。但是,二者对天然水体中硝化作用的相对重要性尚不清楚,有待于进一步深入研究。
4.2. 硝化作用对杏林湾生态环境的影响
根据2005年的统计数据 [13] ,每年排入杏林湾的总氮量为1195~1605 t,假设把水库最上端S1站氨氮占总氮的比例~23%作为输入杏林湾水库的氨氮百分比,则每年输入库区的总氨氮量为275~269 t。再假设本研究测定的夏季和冬季的氨氧化速率的平均值能够代表库区全年的平均氨氧化速率,结合杏林湾水库面积(~2.2 km2)和平均水深(~2.5 m) [12] ,可以估算出杏林湾水库的水体每年通过硝化作用可以将大约142 t氨氮转化为硝氮,相当于输入杏林湾水域氨氮通量的38%~52%。尽管本研究调查站位较少,调查的次数有限,可能导致计算氨氮去除通量存在较大的不确定性,但由此还是可看出,水体的硝化作用对杏林湾氮循环的影响不容忽视。此外,硝化作用需要消耗大量溶解氧,每减少1.0 mol氨需要消耗1.82 mol 氧分子 [20] ,在硝化作用强烈的区域可能造成底层水体或沉积物缺氧而影响其它生物的生长,同时硝化作用是个产生质子的过程,沉积物和水界面强烈的硝化作用,会明显降低沉积物间隙水的pH值,从而导致沉积物中重金属离子的溶出,增加重金属的环境毒性。因此,杏林湾水体的硝化作用对区域氮循环和生态环境演变具有重要意义。
5. 结论
1) 杏林湾水体溶解无机氮和硝化作用强度的空间差异和季节变化显著,湾区上游和下游的溶解无机氮的浓度和硝化速率均比中游高,夏季溶解无机氮的浓度和硝化速率比冬季高,说明溶解无机氮的浓度和温度是控制硝化作用强度空间分布格局和季节变化规律的主要因素。
2) 实验室培养的结果显示,温度、pH、氨的浓度和颗粒物对硝化作用均有重要的影响,其中温度和颗粒物的存在对硝化作用强度影响最为显著。在20℃~30℃之间,温度升高10℃硝化速率可以提高2.9倍;水体中的颗粒物对于硝化作用具有显著的促进作用。
3) 根据研究结果估算出杏林湾水体每年通过硝化作用可以将~142 t氨氮转化为硝氮,相当于输入杏林湾水域氨氮通量的38%~52%,硝化作用对区域氮循环具有重要意义。
基金项目
福建省自然科学基金项目(2015J01167);集美大学科研基金项目。