1. 引言
冷冻干燥技术,又称为真空冷冻干燥技术,是将制品冻结到共晶点温度以下、充分冻结水分后,再在适当的温度和真空度下,利用升华原理去除产品中的水分,获得干燥制品的技术。相对于晒干、喷雾干燥等干燥方式,冷冻干燥技术拥有着诸多优点,使得其在食品、陶瓷、考古等行业的发展日新月异。特别是在生物制品的保存中,冷冻干燥技术已经发挥着不可或缺的作用。
2. 冷冻干燥技术的原理
自然界的水存在固态、液态和气态三种形式,相态的变化与温度、压力密切相关。在标准大气压下,随着温度的升高,固态的冰将融化成液态的水;达到沸点时,液态的水转化成气态的水蒸气。在三相点(图1中O点),固态、液态和气态三相共存,当气压下降至三相点以下时,固态中的水吸热以后,将不融化,直接升华为水蒸气。
冷冻干燥则是利用真空泵将气压维持在较低水平,然后向冻结的制品提供能量,促使水分升华成为水蒸气,然后被冷阱捕获,从而去除产品中的水分 [1] 。
蛋白质、DNA、RNA、代谢产物等生物分子的不稳定源于在溶液中的水解反应 [2] ,因此生物制剂的存储和运输具有非常高的难度。虽然诸多保护成分的加入可以延长液态生物制剂的有效期,但是冷冻干燥技术在生物制品的保存和运输中具有不可替代的地位。
冷冻干燥技术能够除去产品中95%~99%的水分,保证产品可以长期保存而不变质,有效的延长产品的有效期。此外,水分的去除大大减轻了产品的重量,方便产品的长途运输。这些都为稳定性差的生物制品的保存和运输提供了很大的便捷。
相对于晒干、烘干等干燥技术,冷冻干燥在低温下进行,可以保持蛋白、疫苗等热敏性物质的生物活性。
由于冷冻干燥在真空条件下完成,避免了生物制剂在干燥的过程中被氧化而丧失活性。
冷冻干燥时,水以冰晶的形式存在,溶液中的有机盐均匀的分布在物料当中,随着水分逐渐升华离开,溶解物就地析出,避免了其他干燥方式中无机盐随着水分向表面迁移所导致的表面硬化。随着水分的升华,制品逐渐形成疏松多孔的结构,可以实现快速、充分的复溶。
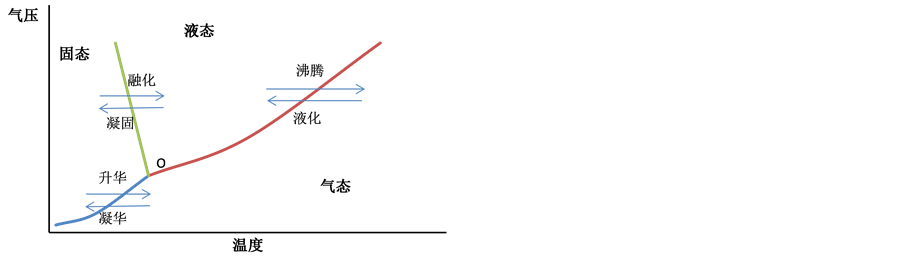
Figure 1. Schematic diagram of phase changes of water
图1. 水的三相变化示意图
3. 冷冻干燥技术工艺
冷冻干燥是通过冻结后升华将水分去除,整个冷冻干燥过程分为预冻、主干燥和解吸干燥三个部分。
3.1. 预冻
溶液冻结温度通常比纯水低,晶体型制剂彻底冻结的温度叫共晶点。在冷冻干燥中,通常需要将制剂在共晶点低10℃~15℃保持1~2小时,以保证制剂被彻底冻结。在预冻中,需要对降温速率进行研究和控制,快速降温可以在缩短预冻阶段时间消耗,但是形成的冰晶较小,增加水蒸气升华时的阻力,导致干燥时间的延长,工艺开发时需针对制剂特点设计降温速率。预冻时,应尽量降低预冻制剂的厚度,以缩短干燥时间,一般分装厚度不要超过15 mm。必要时可采用旋转预冻的方法降低制剂厚度,增大升华表面积。
3.2. 主干燥
主干燥是第一个阶段的干燥过程,制剂中90%水分是在此阶段去除。水分升华的过程需要吸收大量的热量,所以在此过程中需要对产品进行供能加热。但是需要对加热速率进行控制以保证产品的温度在共晶点以下,避免产品温度过高导致产品融化、冻干失败。干燥是在接近真空条件下进行的,气压越低,越有利于升华的发生和水蒸气的逸出,加快干燥速度;但是过低的气压不利于热量的传导,产品难以获得热量而降低了干燥速度。干燥过程中需要对这两方面进行综合考虑,一般将气压控制在0.1~0.3 mbar范围内。
3.3. 解吸干燥
主干燥结束后,制剂中的自由水已经全部升华,剩余~10%的水以结合水的形式残留。在干燥的第二阶段,为了进一步减少产品中的水分,需要对产品进一步加热以促进结合水的升华,即解析干燥。此过程中,可将温度升高至20℃~40℃,真空度进一步增加以促进结合水的解吸附。
4. 冷冻干燥产品配方
冷冻干燥过程是一个复杂的相变过程,其中存在着各种各样的应力,通常包括低温应力、冻结应力(包括枝状冰晶的形成、例子强度的增加、pH值的改变、相分离等)、干燥应力等,这些应力常常是直接或间接导致蛋白质药物不稳定的因素 [3] 。因此需要在冻干制剂中加入一些组分对冻干物质进行保护,这些保护组分按照功能可以分为pH缓冲剂、赋形剂、保护剂、表面活性剂和防腐剂等。
pH缓冲剂则是为了避免在冻结和干燥过程中产生过大的pH变化导致活性损失。工艺开发中需要根据冻干物的性质选择相应的缓冲剂,不适宜的缓冲体系可能导致冻干失败。在天门冬氨酰苯丙氨酸甲酯的冻干中,采用0.1 M的磷酸缓冲体系时,产品的半衰期将从对照组的921天降至98天,增加磷酸盐浓度可进一步将半衰期缩短至77天 [4] 。
当冻干组分的含量少于2%时,冻干产物难以形成固定形状,赋形剂可以在制剂中形成支持基质,避免内容物在冻干过程中逸散,保证制剂在干燥后仍保持理想的外观 [5] 。常见的赋形剂有蔗糖、海藻糖、甘露醇、牛血清白蛋白等。大部分赋形剂在形成分子支架的同时,也扮演保护剂的角色。
在大部分的冻干制剂中,赋形剂和pH缓冲剂还不足以对抗各种应力,所以开发者经常在配方中加入保护剂。优良的保护剂不仅能够在冻干过程中抵抗各种应力,而且能够在贮藏期内一直制品变性。常用的保护剂有多羟基化合物(如甘油、甘露醇、山梨醇等) [6] 、糖类(如蔗糖、乳糖、海藻糖) [7] 、氨基酸(如甘氨酸、赖氨酸、丙氨酸) [8] 、多聚物(如聚乙二醇、明胶、聚乙烯亚胺) [9] 、蛋白质(如牛血清白蛋白、人重组白蛋白) [10] 等。保护剂的保护机理主要有两种理论:“水替代”假说和“玻璃态”假说。“水替代”假说认为,溶液中的蛋白质等生物分子周围包裹着一层水分子膜,与溶质分子形成氢键,保护着溶质分子的结构和功能。在干燥的过程中,水分子膜的破坏导致溶质结构不可逆改变,冻干保护剂可以替代水分子与溶质形成氢键,使其在缺水条件下仍维持原有结构,保持活性 [11] 。“玻璃态”假说认为,糖类、多羟基化合物和蛋白质等均能形成玻璃态,玻璃态兼有固体和液体的性质,其黏度可以高达1012 Pa·s。在冷冻干燥的过程中,保护剂在生物分子表面形成一层玻璃态分子保护层,由于黏度很高,扩散系数很低,蛋白质等分子的运动大大被限制,分子构象互变及构象松弛减慢,从而维持了分子的结构和功能 [12] 。
吐温和Triton等表面活性剂在制剂的干燥和储存的过程中可以避免生物分子的之间非特异性结合,减少生物分子聚集沉降速率。同时,表面活性剂还可以促进制品快速充分复溶,所以冻干制剂中也常常加入表面活性剂。
为了提高冻干产品的有效期,冻干制剂中常加入防腐剂以抑制微生物的生长,常用的防腐剂有叠氮化钠、proclin等。
5. 冷冻干燥机的选择
为了精确的控制冷冻干燥过程中的气压和温度,其过程均在特殊的仪器中(冷冻干燥机)完成。目前市场上的冷冻干燥机型号繁多、配置各异。每个型号都适用于特定的场合和工艺要求。选择合适的冷冻干燥机可以有效缩短冻干周期、降低能耗;相反,选择错误的冻干机型号有可能直接导致样品报废,甚至损坏冻干机。冷冻干燥机主要包括干燥腔、冷阱和真空泵组成。
干燥腔的主要作用是盛放样本,为冷冻干燥提供一个密闭的空间。选择干燥腔时,需要保证足够的空间以容纳生产批量;根据冻干时的容器选择相应的干燥腔,容器为冻干瓶时需要选择带冻干瓶接口的干燥腔,冻干完成后需要对产品进行封装则应选择配置了压盖或者安瓿瓶熔封装置的干燥腔;透明的干燥腔有利于观察,但光线的照射不利于精确的供能控制;部分型号的干燥腔还配置有产品温度、产品电阻监测装置,可以根据需求进行选择。
冷阱是升华的水蒸气凝华被捕获的地方,它的选择对于冷冻干燥至关重要。选择冷阱主要依据最低温度和集冰量两个参数进行。冷阱的温度需要保持在制剂共晶点10℃~15℃以下,才能保证充分捕获水分。集冰量是指冷阱能够捕获多少溶剂,冻干过程中,冷阱的最大集冰量最好能够达到溶剂的3倍以保证干燥的充分进行。此外,如果干燥物质具有腐蚀性,则需选择耐腐蚀材质的冷阱。
真空泵的作用是及时将干燥腔内的气体抽出,维持干燥腔内的真空度。选择真空泵主要考虑其抽气速度和极限真空度是否符合工艺要求。
6. 展望
冷冻干燥技术拥有种种优势,但是其局限性仍制约了它的广泛应用。冷冻干燥机价格昂贵、操作难度较高,使得技术开发的投入很高;冷冻干燥周期长、能耗高,大大提升了使用成本;冷冻干燥技术过程复杂,控制点繁多,工艺开发对于时间和人员都有较高的要求,这些因素都限制了冷冻干燥技术的推广。
然而,在蛋白质等生物制品的干燥保存方面,尚无比冷冻干燥更加易用、有效的技术。而随着冷冻干燥技术在理论研究飞速的进展,冷冻干燥设备和技术已经趋于完善。现代冷冻干燥设备不仅能满足各种冻干工艺的加工要求,在操作和控制上也逐渐实现了智能化;随着冻干机技术的进步,冷冻干燥对于能量的利用率不断提升,冻干周期也显著缩短;真空冷冻干燥技术与自动加塞技术、安瓿瓶自动熔封技术的结合促进了其在生物医药的广泛使用。新的保护剂的开发和使用大大的降低冷冻干燥工艺的开发难度,缩短了开发周期。计算机技术在冻干过程中的应用使得能够建立相应的数学模型对冻干过程中物料的真实状态进行模拟解析,从而也使得冻干技术得以逐渐完善和提高。这些方面的进步和完善将在生物制剂的保存上赋予冷冻干燥技术广阔的发展空间。