1. 引言
近年来,由于便携式电子器件以及电动汽车的市场需求呈现指数级的增长,因此,要求创造出轻便,紧凑的高能量密度和功率密度的储能器件,以满足不断增长的需求量 [1] [2] [3] 。这其中,电化学超级电容器因具有高比功率和长寿命等突出优点,近年来已成为电化学储能领域的研究热点 [4] [5] 。
活性炭由于高比表面积,优异的导电性已经被广泛的用作超级电容器的电极材料,其存贮机制是基于电极/电解液电荷存储的双电层原理(EDLCs),决定EDLCs电容性能的因素有活性炭的有效比表面积以及最佳孔径(与电解液离子半径相匹配) [6] [7] 。研究表明微孔-介孔碳材料其相互连通的介孔更有利于提高电荷存储密度和缩短离子迁移距离。因此,具有微孔-介孔复合多孔碳由于具有杰出的电容性质而引起了研究者的极大关注。
迄今为止,合成微孔-介孔碳材料常规采用模板法 [8] [9] [10] [11] [12] ,但这种模板技术步骤多且繁琐,甚至有时需要环境不友好的条件下进行。因此,探求多孔碳的多种制备方法,对于发展高性能超级电容器具有重要意义。生物质基多孔碳已经展现出作为电极材料在超级电容器方面的优势 [13] ,由于生物质材料具有来源丰富、可再生、简易并环境友好等特点,并且以生物质为前驱体所制备的生物质炭不仅具有独特的复杂多级形貌结构,而且具有发达的孔隙结构,高的比表面积和丰富的表面官能团,因此,多种生物质,如海藻,椰子壳等 [14] [15] [16] [17] [18] 已经作为碳源被研究用于发展高级炭材料。更重要的是,对比逐渐萎缩的化石能源,利用可再生的生物质发展碳基能源材料是一种环保可持续性的方法。
本文以大连黄海黑石礁岸边拾取的新鲜海带为原料,通过筛选、干燥、预处理以及高温炭化的过程制备微孔/介孔复合多级孔炭材料,并通过控制焙烧温度,提高材料的比表面积,从而提高材料的比电容。电化学测试表明海带基生物质多孔炭材料表现出良好的电化学性能,800℃焙烧下的炭电极在电流密度5 A/g下,比电容达到200 F/g,循环5000次,电容几乎无衰减,表现出极佳的稳定性。
2. 实验部分
2.1. 实验材料
氢氧化钾(KOH,国药集团化学试剂有限公司,AR);盐酸(HCl,天津市科密欧化学试剂有限公司,优级纯);PVDF(国药集团化学试剂有限公司,AR);乙炔黑(山西卡本贸易有限公司);1-甲基-2-吡咯烷酮(NMP,国药集团化学试剂有限公司,CP);泡沫镍(爱蓝天高新技术材料(大连)有限司)。超级电容器用商业活性炭YP50F购于日本Kurary公司。
2.2. 实验过程
2.2.1. 海带预处理及碳化
选取的新鲜海带经超声波清洗后置于80℃的恒温干燥箱中干燥。然后将干燥的海带放入高温管式炉中,N2为载气(流速为150 mL/min)进行高温焙烧,升温速率为10℃/min,从室温分别升至700℃、800℃、900℃,并恒温1小时,随后自然冷却至室温,产物研磨后过筛,最后得到样品。样品根据焙烧温度相应标记为H700、H800、H900。
2.2.2. 电极的制备及电化学性能测试
电极制作:将海带基活性炭粉末与PVDF溶液(溶剂为N甲基吡咯烷酮,浓度为5% (wt))、乙炔黑按质量比90:5:5混合均匀成糊状,将糊状物均匀涂抹到面积为1 × 1 cm2泡沫镍上,放入真空干燥箱中120℃恒温干燥过夜,压片机10 MP下压片制作成电极,然后,放入浓度为4 M的KOH溶液中,真空条件下浸泡12小时至电解液完全浸润电极材料。商业活性炭的电极制作过程同海带基活性炭。
电化学测试:测试体系为三电极体系,工作电极为活性炭电极,参比电极为汞/氧化汞电极,对电极为铂片电极(3 × 2 cm2)。电解液为4 M的KOH溶液。对电极进行循环伏安、交流阻抗、恒流充放电测试。循环伏安和恒流充放电电压区间均为−1.0~0 V。
2.3. 材料表征
采用扫描电子显微镜(SEM, JSM-7800F JEOL Co., Japan)对炭材料的形貌进行表征。元素分析采用X射线光电子能谱仪(XPS, Thermo ESCALAB 250Xi),射线源为AlKα射线,工作电压12 kV,电流10.8 mA。炭石墨化程度使用拉曼光谱仪(Renishaw)测试,Ar离子激光器,发射波长为532 nm,炭粉末样品直接放置于玻璃载玻片上测试。N2吸附/脱附等温线采用Quantachrome QuadraWin吸附仪在77 K条件下获得,样品的比表面积采用Brunauer-Emmett-Teller (BET)方法计算;孔容和孔径由等温线吸附分支采用Barrett-Joyner-Halenda (BJH)模型计算;微孔表面积(Sm)采用V-t plot方法计算。
3. 结果与讨论
3.1. 元素分析测试(XPS)
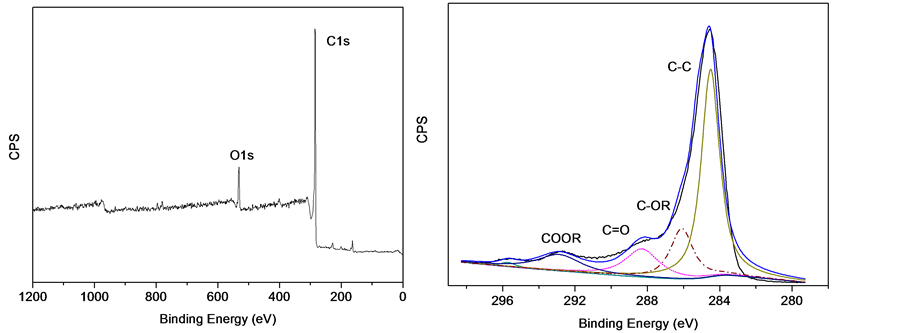
H800样品的XPS谱图及C1s峰谱分析如图1所示。由图可知,海带基多孔碳主要以C-C结合的石墨碳为主(284.7 ± 0.2 eV),且含有少量的内酯基(C-OR),羰基(C=O)和羧基(COOR),这三种基团的电子结合能分别为286.1 ± 0.2 eV 、288.1 ± 0.2 eV和292.5 ± 0.2 eV。此外,材料表面还含有少量的N元素。H700、H800、H900样品的O、N元素及含氧官能团的相对含量见表1,从表中可以看出,随着热处理温度的升高,O、N及含氧官能团含量均有所下降。
 (a)(b)
(a)(b)
Figure 1. (a) XPS analysis of H800, (b) High-resolution XPS analysis of the C 1s peak of H800
图1. (a) H800XPS谱图,(b) H800C1s峰的高分辨XPS谱图

Table 1. XPS surface elemental characterization of the as-received seaweed carbons
表1. 海带基炭表面元素XPS分析
3.2. 形貌分析
图2(a)为在岸边采集的新鲜海带的数码照片,图2(b)~(d)为不同焙烧温度的炭微观结构,从图中可以看出经过焙烧后得到的活性炭均为多孔结构。为进一步分析孔径和孔结构,采用N2吸脱附等温线进行了表征。
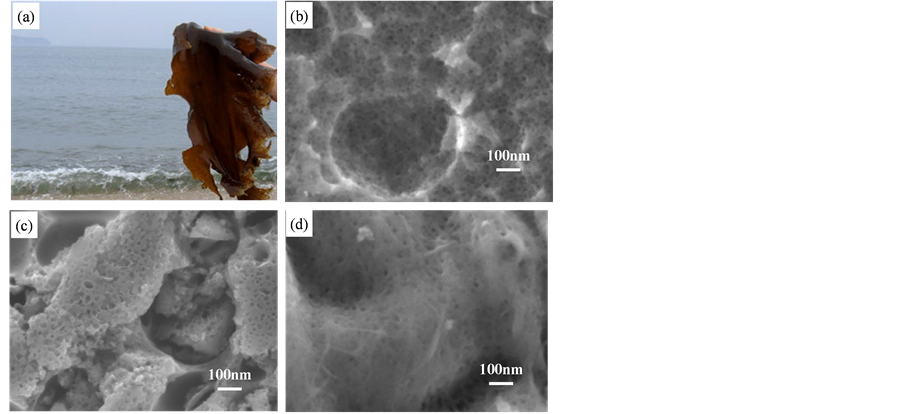
Figure 2. (a) Digital picture of the seaweed; SEM images of seaweed-derived carbon materials: (b) 700˚C (H700), (c) 800˚C (H800), (d) 900˚C (H900)
图2. (a) 海带光学照片,不同温度处理下的海带基碳材料SEM图:(b) 700℃ (H700),(c) 800℃ (H800),(d) 900℃ (H900)
3.3. N2吸脱附等温线
图3(a)是样品的氮吸附脱附等温线,从图中可以看出,所有样品的氮吸附脱附等温线均为第IV型吸附等温线,该类曲线表明样品有丰富的介孔存在 [19] 。等温线在相对分压0.5~0.7之间出现了毛细凝聚现象引起的滞后环,对应海带基介孔炭的介孔结构,H800和H700的滞后环大于H900,且朝相对分压较大的方向偏移。经计算得出,H700,H800,H900三种样品的比表面积分别为:1606.96 m2/g,1703.97 m2/g,1285.78 m2/g,由此可见H800的比表面积最大。样品的孔径分布如图3(b)所示,从图中可以看出,样品的孔径分布在1.2~4 nm区间,为微孔-介孔复合结构。孔结构参数如表2所示。从表中可以看出,样品的微孔孔容率、微孔面积率随热处理温度的升高都有不同程度的增加,H800的总孔容最大,结合微孔-介孔复合结构有利于获得高比电容和优良的倍率性能。
 (a)(b)
(a)(b)
Figure 3. Pore characterization of seaweed-derived porous carbon. (a) Nitrogen adsorption desorption isotherms on the carbon, (b) Pore size distributions calculated using a slit-pore DFT model
图3. 海带基多孔炭的孔特征。(a) N2吸附脱附等温线,(b) 采用Slit-pore DFT模型计算的孔径分布曲线

Table 2. Pore textural parameters of samples
表2. 样品的孔结构参数
3.4. Raman图谱分析
从图4的拉曼光谱中,可以看出三个样品在1360 cm−1 (D峰)和1595 cm−1 (G峰)处都有明显的特征峰,其中,D峰代表晶体缺陷和不规则性,G峰代表六方晶系的炭原子平面。即这两个峰的相对强度(ID/IG)与炭材料中的缺陷位成正比 [20] 。经计算,H700、H800和H900的ID/IG之比分别1.12、1.03、0.86,这说明随着焙烧温度的增加炭的缺陷位减少,石墨化程度得到提高。
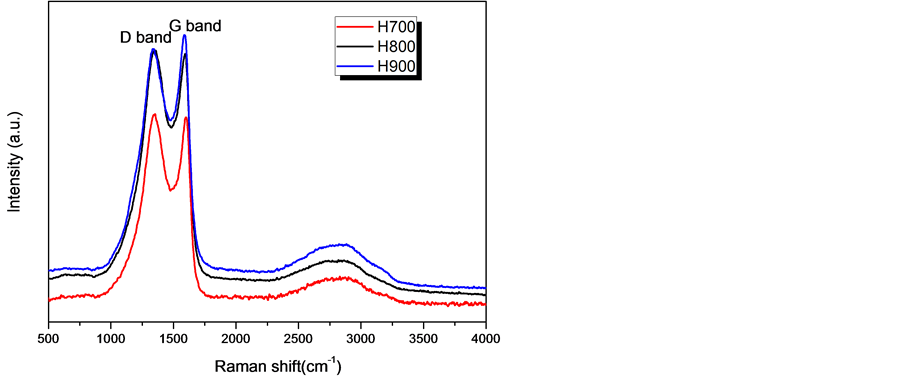
Figure 4. Raman spectroscopy of the samples
图4. 样品拉曼光谱图
3.5. 电化学性能分析
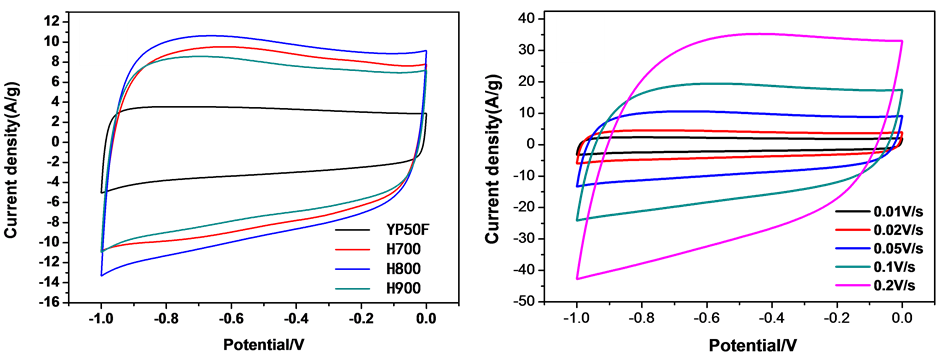
为了研究海带基炭电极的电化学性能,对组装的电极进行循环伏安、恒流充放电测试。图5(a)为商业活性炭YP50F,H700,H800和H900在50 mv/s扫描速率下的循环伏安曲线。从图中可以看出,YP50F的CV曲线表现出标准的矩形特征,这也验证了商业化的YP50F具有很好的导电性,适合做电容器用 [21] [22] 。H700、H800、H900三种材料的循环伏安曲线在该扫描速率下也均呈现准矩形的特征, 展示出双电层电容特性,同时,在−0.8~−0.5V区间出现了微弱的氧化还原峰,由XPS元素分析可知这是由于活性炭表面具有含氧官能团等杂原子,因此,活性炭也具有一定的赝电容特征,但电容贡献中主要以双电层存储电荷为主,而以杂原子产生的氧化还原反应微弱,赝电容效应并不明显。在所有材料中,YP50F的CV面积最小而H800的面积最大,这表明H800具有更大的比电容。随后,对H800在多种扫描速率下的可逆性进行了表征,结果如图5(b)所示。图5(b)为H800在10 mv/s到200 mv/s扫描速率下的循环伏安曲线。从图中可以看出,随着扫描速率的增加,循环伏安曲线的矩形度没有发生显著变化,仍保持良好的矩形的特征,表明800℃下处理的海带基炭作为电极材料表现出良好的可逆性。进一步采用面积比电容公式:
 (1)
(1)
式中: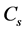 为比电容(F∙g−1);
为比电容(F∙g−1);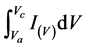 为CV曲线面积(V∙A);m为电极片中活性物质的质量(g);
为CV曲线面积(V∙A);m为电极片中活性物质的质量(g);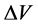 为充放电电压范围(V);
为充放电电压范围(V);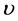 为扫描速率(V/s)。计算了不同扫速条件下电极材料的面积比电容,如图5(c)所示。从图中可以看出,YP50F的比电容最小,其它三种电极的比电容均好于YP50F。在所有的电极中,H800的比电容表现最佳。
为扫描速率(V/s)。计算了不同扫速条件下电极材料的面积比电容,如图5(c)所示。从图中可以看出,YP50F的比电容最小,其它三种电极的比电容均好于YP50F。在所有的电极中,H800的比电容表现最佳。
样品的恒流充放电性能如图6所示。图6(a)为样品在电流密度为2 A/g下的充放电曲线,从图中可以看出,四种电极在该电流密度下,充放电曲线均呈现对称三角形,电压与放电时间呈线性关系,表现出良好的双电层电容特性和在充放电过程较好的可逆性。此外,随着热处理温度的升高,电压随时间变化的线性度有所增加,说明高温处理后的电极材料表现出较好的电荷转移的可逆性。图6(b)是H800在不同电流密度下的充放电行为图,从图中可以看出,低电流密度下充放电过程所用时间更长,在充放电过程中需要充分的插入或者释放,随着电流密度的逐渐增大,材料的充放电过程的时间缩短,放电电压与时间保持良好的线性关系,表明电极材料具有良好的可逆性。根据电极材料的质量比电容公式:
 (a) (b)
(a) (b) (c)
(c)
Figure 5. (a) CV curves of YP50F, H700, H800, and H900 at scan rate of 50 mV/s, (b) CV curves of H800 at scan rate of 10, 20, 30, 50, 100 and 200 mV/s, (c) specific capacitance curves of YP50F, H700, H800, and H900 at different scan rate
图5. (a) YP50F, H700, H800, H900在50 mV/s下的CV曲线图,(b) H800在10, 20, 30, 50, 100和200 mV/s扫速下的CV曲线图,(c) YP50F, H700, H800和H900在不同扫描速度下的比电容曲线图
 (a) (b)
(a) (b)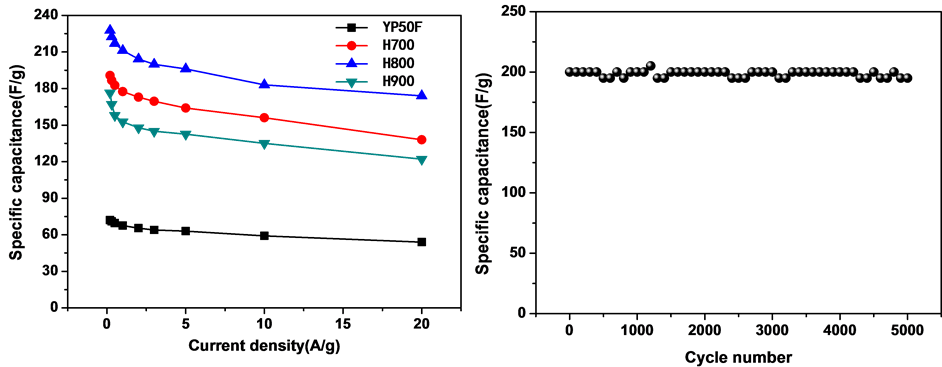 (c) (d)
(c) (d)
Figure 6. GCD curves of (a) YP50F, H700, H800, and H900 at 2 A/g current density, (b) H800 at different current density, (c) specific capacitance curves of YP50F, H700, H800, and H900 at different current densities, (d) Cycle performance of H800 at a current density of 5 A/g
图6. (a) YP50F、H700、H800、H900在电流密度为2 A/g下的充放电曲线,(b) H800在不同电流密度下的充放电曲线,(c) YP50F, H700, H800, H900在不同电流密度下的比电容曲线;(d) H800在5.0 A/g电流密度下的循环性能
 (2)
(2)
式中: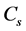 为比电容(F∙g−1);I为充放电电流(A);
为比电容(F∙g−1);I为充放电电流(A);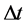 为放电时间(s);m为电极片中活性物质的质量(g);
为放电时间(s);m为电极片中活性物质的质量(g);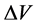 为充放电电压范围(V)。计算样品的比电容,与电流密度关系如图6(c)所示,所有样品均表现出比电容随着电流密度的增大而减小的性质,与图5(c)趋势一致。在相同电流密度下,H800表现出更大的比电容,这说明H800具有更优异的电容特性。这是由于两方面原因:一方面,H800具有微孔-介孔的复合孔结构和大的比表面积,不仅表面与电解液充分接触,而且也有利于电解液渗入到电极材料的内部空间,使得电极材料与电解液接触更为充分。另一方面,本实验中采用的水系KOH电解液的OH−和K+的有效离子尺寸分别为3.62和4.2 Å [23] ,这于N2分子的尺寸(~4 Å)接近,因此,吸附N2的孔隙也可以吸附水合离子,这样几乎所有的表面都能形成有效的双电层电容,从而更大程度上发挥活性炭比表面积所起的作用。图6(d)为样品在电流密度为5 A/g下的循环稳定性曲线,从图中可以看出电极在5000次循环后,比电容无衰减,表现出非常好的稳定性。
为充放电电压范围(V)。计算样品的比电容,与电流密度关系如图6(c)所示,所有样品均表现出比电容随着电流密度的增大而减小的性质,与图5(c)趋势一致。在相同电流密度下,H800表现出更大的比电容,这说明H800具有更优异的电容特性。这是由于两方面原因:一方面,H800具有微孔-介孔的复合孔结构和大的比表面积,不仅表面与电解液充分接触,而且也有利于电解液渗入到电极材料的内部空间,使得电极材料与电解液接触更为充分。另一方面,本实验中采用的水系KOH电解液的OH−和K+的有效离子尺寸分别为3.62和4.2 Å [23] ,这于N2分子的尺寸(~4 Å)接近,因此,吸附N2的孔隙也可以吸附水合离子,这样几乎所有的表面都能形成有效的双电层电容,从而更大程度上发挥活性炭比表面积所起的作用。图6(d)为样品在电流密度为5 A/g下的循环稳定性曲线,从图中可以看出电极在5000次循环后,比电容无衰减,表现出非常好的稳定性。
4. 结论
本文采用黄海大连黑石礁海域岸边拾取的新鲜海带作为炭电极基材,通过一系列过程成功制备了具有微孔/介孔复合结构的超级电容器用多孔炭电极材料。结构分析测试表明,不同焙烧温度下炭电极的结构和比表面积有所差异,800℃ (H800)的BET最大为1703.97 m2/g。电化学测试结果表明海带基炭电极性能明显好于商业活性炭,800℃下的炭电极在5 A/g下比电容高达200 F/g,且循环5000次比电容无衰减。这说明海带基多孔炭电极材料具有优良的电化学性能,有望在商业化超级电容器活性炭电极中得到应用。
致谢
感谢辽宁省自然科学基金(批准号: ) ),辽宁省教育厅基金(批准号:L201617),中国博士后科学基金(批准号:2014M551138)和大连海洋大学“蔚蓝英才”基金(批准号:500210043)的支持。
*通讯作者。