1. 引言
氧化锌烟灰中氟、砷的去除一直是冶金工作者关注的热点问题 [1] [2] [3] [4] 。目前国内外许多厂家采用多膛炉和回转窑焙烧除氟 [5] ,也有研究报道采用水洗法或碱洗法脱氟 [6] ,碱洗法的氟脱除效果较好,然而物料经碱洗后还需进行水洗脱碱,废水量大。此外,有研究报道在湿法浸出液中除氟,如魏昶 [7] 等采用萃取法除溶液中的氟,谢维新 [8] 采用氟化钙沉淀法去除硫酸锌电解液中氟。
含砷氧化锌烟灰中脱砷的方法主要有焙烧脱砷 [9] 和浸出脱砷 [10] 。焙烧法中砷在高温下以三氧化二砷的形态挥发从而实现分离,该方法原料适用范围小、对环境污染严重。浸出脱砷是烟灰在浸出剂中选择性溶出,使砷从固相转化为液相,最后在液相中加以脱砷的方法。常用的脱砷剂有硫化钠 [11] 、硫酸亚铁 [12] 和偏钛酸 [13] 等。
本研究提出采用氨-氯化铵溶液浸出氧化锌烟灰,氯化铁作为除砷剂,氯化钙作为除氟剂,经净化除杂、加酸沉锌、水解转化和煅烧等工序制备活性氧化锌,以期获得高的锌直收率和高品质的活性氧化性,为从氧化锌烟灰中生产活性氧化锌提供了一种新的工艺路线。
2. 实验
2.1. 原料和试剂
本研究所采用的主要原料是某冶炼厂的氧化锌烟灰,其主要化学组成见表1。实验所用到的试剂主要有氯化铵、氨水、盐酸、三氯化铁、锌粉、氯化钙和粉末状活性炭。
2.2. 实验流程及方法
图1为从氧化锌烟灰制取活性氧化锌的工艺流程。该工艺流程主要包括浸出过程,采用FeCl3除砷、CaCl2除氟、锌粉置换除杂和活性炭吸附等四段净化过程,净化液加酸沉锌以及沉锌物两次水解转化和煅

Table 1. The main chemical composition of zinc oxide fume dusts
表1. 氧化锌烟灰的主要化学组成

Figure 1. The process of preparing active zinc oxide from zinc oxide fume dusts
图1. 从氧化锌烟灰制取活性氧化锌的工艺流程
烧等过程。工艺流程中的净化液加酸沉锌母液和一次水解转化母液经蒸发浓缩可返回浸出,二次水解转化母液经适当处理即可达标排放。
浸出过程系将一定量的浸出剂加入反应烧杯中,加热至50℃,加入氧化锌烟灰进行浸出,搅拌时间1 h后过滤,得到浸出液和浸出渣。
除砷过程则按不同的铁砷摩尔比向浸出液中加入FeCl3,在室温下搅拌反应30 min,除去浸出液中的As;过滤后进行除氟,除氟过程是在滤液中加入一定量的氯化钙,在室温下搅拌反应1 h,除去浸出液中的F。过滤后进行锌粉置换除杂,在40℃下,锌粉投加量为5 g/L,搅拌反应1 h,置换除去浸出液中Pb、Cu、Cd等重金属杂质离子;过滤后进行下一步活性炭吸附有机物,即在滤液中加入3 g/L的活性炭,在室温下搅拌吸附1 h,以除去溶液中残留的有机物,过滤所得滤液进入下一步加酸沉淀过程。
加酸沉淀过程系在室温下在除氟液中缓慢加入12 mol/L的盐酸,以调节溶液的pH为6.7进行加酸沉淀,搅拌一段时间后过滤。滤渣经洗涤、干燥后得到Zn(NH3)2Cl2。滤液返回浸出使用。
水解转化分为两步进行,一次水解转化系将Zn(NH3)2Cl2和水按5:1液固比进行水解转化,反应温度为80℃,反应时间为1 h。经过滤、洗涤、干燥得到Zn(OH)1.6Cl0.4。二次水解转化系将Zn(OH)1.6Cl0.4和水按5:1液固比进行水解转化,反应温度为80℃,反应时间为1 h。经过滤、洗涤、干燥得到Zn(OH)2。将Zn(OH)2置于马弗炉中,500℃煅烧2 h,得到活性氧化锌。一次水解转化滤液经蒸馏浓缩后返回浸出,馏出液返回水解转化用。
2.3. 分析方法
采用X射线荧光光谱仪(XRF)测定氧化锌烟灰和浸出渣的主要化学组成;采用EDTA滴定法测定浸出液中锌的含量;采用X射线衍射仪(XRD)测定浸出渣、沉淀产物、水解转化产物和煅烧产物的物相;采用ICP-OES测定净化前后溶液中Pb、Cu、As、Cd、F杂质元素的含量。
3. 试验结果与讨论
3.1. 浸出过程
图2、图3分别为NH4Cl和NH3·H2O浓度对烟灰中Zn、Cd、Cu、Pb、F浸出率的影响。从图2可以看出,Zn、Cd、Cu、Pb浸出率随着NH4Cl浓度的增大而升高,当NH4Cl浓度大于2 mol/L时,Zn浸出率变化不大,均能达到90%以上,当NH4Cl浓度大于4mol/L时,Pb浸出率才开始增加;而F浸出率随着NH4Cl浓度的增大则逐渐降低。从图3可以看出,随着NH3·H2O浓度的增加,烟灰中Zn、Cd、Cu、F浸出率随之提高,而Pb浸出率随之降低;当NH3·H2O浓度大于2 mol/L时,Zn浸出率变化不大。故本研究选择NH3·H2O浓度为2 mol/L,NH4Cl浓度为4 mol/L。
表2为氧化锌烟灰在NH4Cl浓度4 mol/L、NH3·H2O浓度2 mol/L、浸出温度50℃、浸出时间1 h的浸出条件下浸出渣的化学组成,浸出渣率为20.09%。由表2可知,浸出渣主要由Pb、Cl、Zn等元素组成。对比表1与表2,大部分Zn进入到浸出液中,同时少量Pb、Cu、F、As、Cd等杂质也进入到了浸出液中。图4为浸出渣的XRD图谱,其物相主要为Pb(OH)Cl和少量的Zn(NH3)2Cl2。
3.2. 净化过程
图5为FeCl3除砷时铁砷摩尔比(nFe/nAs)对除砷率的影响。从图5中可以看出,除砷率随着nFe/nAs增
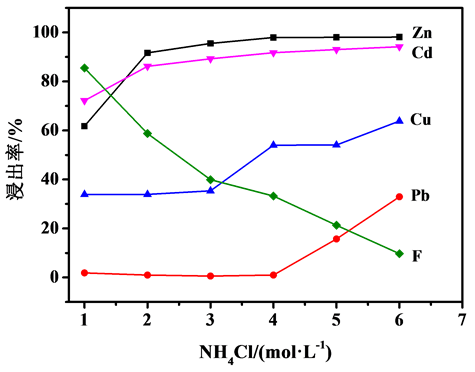
Figure 2. Effect of NH4Cl concentration on leaching rate of fume dusts, reaction temperature = 50˚C,reaction time = 1 h
图2. NH4Cl浓度对烟灰浸出率的影响,反应温度50℃,反应时间1 h

Table 2. The main chemical composition of leaching residue
表2. 浸出渣的主要化学组成
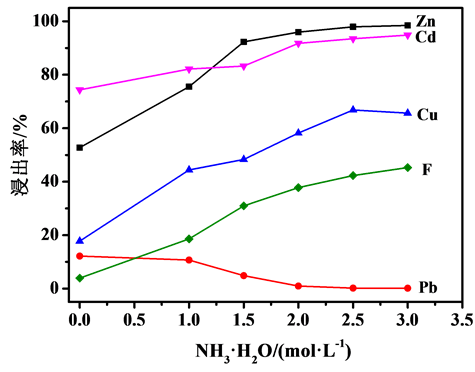
Figure 3. Effect of NH3·H2O concentration on leaching rate of fume dusts, reaction temperature = 50˚C,reaction time = 1 h
图3. NH3∙H2O浓度对烟灰浸出率的影响,反应温度50℃,反应时间1 h

Figure 4. X-ray diffraction pattern of leaching residue
图4. 浸出渣的XRD图
大而增大。当nFe/nAs = 2时,除砷率大于90%,溶液中残余砷浓度为58 mg/L;当nFe/nAs = 5时,除砷率达到99.7%以上,溶液中残余砷浓度为2.3mg/L;继续增大nFe/nAs对除砷率影响不大。
图6为CaCl2除氟时钙氟摩尔比(nCa/nF)对除氟率的影响。从图6中可以看出,随着氯化钙加入量的增加,除氟率显著升高。当nCa/nF = 0.5时,除氟率为89.2%,溶液中残余氟离子浓度为71.1 mg/L;当nCa/nF = 1时,除氟率达到98.5%以上,溶液中残余氟离子浓度为9.5 mg/L;继续增加氯化钙的加入量,溶液中氟离子浓度的降幅不大。

Figure 5. Effect of molar ratio of ferric to arsenic on As removal, reaction temperature = 25℃, reactiontime = 30 min
图5. 铁砷摩尔比对除砷率的影响,反应温度25℃,反应时间30 min

Figure 6. Effect of molar ratio of calcium to fluorine on F removal, reaction temperature = 25℃, reactiontime = 1h
图6. 钙氟摩尔比对除氟率的影响,反应温度25℃,反应时间1 h
表3为浸出液经除砷、除氟以及锌粉置换除杂和活性炭吸附等净化工序后溶液中杂质的浓度。由表3可知,本研究采用的净化工艺可有效地除去浸出液中Pb、Cu、Cd、As、F等杂质,有利于后续制备高品质氧化锌。
3.3. 沉淀过程
图7为经净化处理后的浸出液加酸沉淀产物的XRD图谱。从图7中可以发现几乎所有的峰都对应于Zn(NH3)2Cl2的特征峰,没有其他明显的杂峰,说明产物为单一物相Zn(NH3)2Cl2。从沉淀过程的试验现象可以看出,当溶液的pH降低至8.0时,开始析出沉淀;继续降低溶液的pH,沉淀析出量快速增加;当pH接近中性时,沉淀析出量增加幅度逐渐减缓;当溶液的pH = 6.70时,Zn(NH3)2Cl2沉淀的析出率达到最大,约占溶液中总锌的82.85%,即可将溶液中82.85%的锌得到有效的分离。当继续降低溶液的pH时,沉淀物快速溶解;当溶液的pH = 5.75时,Zn(NH3)2Cl2沉淀完全溶解消失。

Table 3. The concentration of impurities in the solution after purifications (mg/L)
表3. 净化后溶液中杂质的浓度(mg/L)
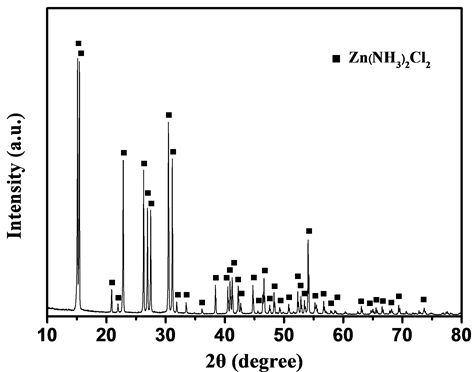
Figure 7. X-ray diffraction pattern of the precipitation
图7. 沉淀产物的XRD图

Figure 8. X-ray diffraction pattern of the first hydrolysis product, the liquid-solid ratio = 5:1, reaction temperature = 80˚C, reaction time = 1 h
图8. 一次水解转化产物的XRD图,液固比 = 5:1, 反应温度80℃,反应时间1 h
3.4. 水解转化制备氧化锌
图8为Zn(NH3)2Cl2在液固比5:1、反应温度80℃、反应时间1 h的水解条件下一次水解转化产物的XRD图。从图8中可以看出,一次水解转化产物中未出现明显Zn(NH3)2Cl2的特征峰,其物相主要为Zn(OH)1.6Cl0.4。图9为在液固比5:1、反应温度80℃、反应时间1 h的水解条件下对Zn(OH)1.6Cl0.4进行二次水解转化产物的XRD图。从图9中可以看出,Zn(OH)1.6Cl0.4已完全转化为Zn(OH)2,其中还出现了少量ZnO的特征峰,这可能是由于Zn(OH)2脱水分解为ZnO所致。

Figure 9. X-ray diffraction pattern of the secondary hydrolysis product, the liquid-solid ratio = 5:1, reaction temperature = 80˚C, reaction time = 1 h
图9. 二次水解转化产物的XRD图谱,液固比= 5:1, 反应温度80℃,反应时间1 h
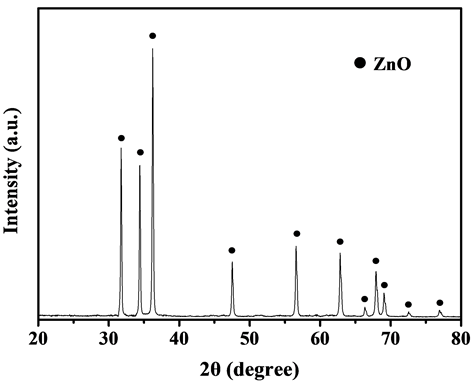
Figure 10. X-ray diffraction pattern of the preparative active zinc oxide, calcination temperature = 500˚C, calcination time = 2 h
图10. 活性氧化锌的XRD图,煅烧温度500°C,煅烧时间2 h
图10为二次水解转化产物经500℃煅烧2 h后所得活性氧化锌的XRD图。从图10中可以看出,其衍射峰与氧化锌的特征峰完全一致,说明本工艺制备的活性氧化锌纯度很高,无杂质相存在。
表4为所制备的活性氧化锌产品指标和HG/T2572-2012标准指标的比较。由表4可知,本研究所制备的产品质量各项指标均达到标准指标。
4. 结论
采用氯化铵和氨水浸出氧化锌烟灰,浸出液经四段净化后,通过加酸沉锌、水解转化和煅烧,制备得到活性氧化锌。浸出反应最佳条件:氯化铵浓度4 mol/L,氨水浓度2 mol/L,浸出温度50℃,浸出时间1 h,Zn浸出率达到98%以上;净化反应最佳条件:铁砷摩尔比5:1,钙氟摩尔比1:1;加酸沉锌最佳pH为6.7,得到Zn(NH3)2Cl2(s)。水解转化反应最佳条件:一次水解转化液固比5:1,反应温度80℃,反

Table 4. The comparison of active zinc oxide product indexes prepared by experiment with standard indexes
表4. 实验制备的活性氧化锌产品指标与标准指标的比较
应时间1 h,得到Zn(OH)1.6Cl0.4;二次水解转化液固比5:1,反应温度80℃,反应时间1 h,得到Zn(OH)2,经干燥,500℃煅烧2 h,得到活性氧化锌,产品质量各项指标均达到HG/T2572-2012标准指标,锌的直收率达80%以上。此工艺为从氧化锌烟灰中生产活性氧化锌提供了一种新的工艺路线,有关氨浸出液加酸沉淀机理和水解转化机理,还需进一步深入研究。