1. 引言
丝胶是蚕丝的主要成分之一,占蚕丝组分含量的25%~30% [1] 。丝胶由18种氨基酸组成(其中有8种是人体必需氨基酸),含有羟基、羧基以及氨基等具有较强极性侧基的氨基酸约占总量的70% [2] ,因此丝胶是一种水溶性球状蛋白 [3] 。丝胶主要来源于茧壳和生丝、丝绸工业产生的废水和5龄幼虫解剖取出的中部丝腺 [4] 。丝胶的分子量超过200kDa,并且表现出层状结构 [5] 。目前被广泛认同的是,来自蚕茧的SS和从中间丝腺提取的天然SS包含无规卷曲和β-折叠,分别对应于无定形和结晶区域 [6] [7] ,以及一些β-转角,但在大多数情况下没有α-螺旋 [8] 。
虽然丝胶一直作为丝绸工业的副产品随废水排放,但由于其一系列的优良特性,近些年的研究表明丝胶蛋白在生物医学和化妆品应用领域有极大的潜力。大鼠在口服丝胶蛋白后血液中的胰岛素水平显著增加 [9] 。丝胶蛋白作为一种抗氧化剂,通过抑制过氧化氢诱导的氧化应激表现出抗氧化能力 [10] 。丝胶可以促进大鼠皮肤伤口和角膜上皮细胞的愈合 [11] 。以京尼平作交联剂的sericin-PVA三维支架也用于组织工程人工皮肤 [12] 。陈忠敏等 [13] 添加壳聚糖和甘油磷酸钠到丝胶中形成温敏性复合凝胶用于肝脏组织工程支架。
迄今为止,凝胶化行为已经被广泛研究 [14] [15] 。凝胶化归因于丝胶蛋白的构象变化。丝胶蛋白的凝胶化导致β-折叠结晶,同时,凝胶化程度决定了β-折叠构象的丝胶蛋白的形成量 [16] 。然而,丝胶蛋白凝胶表现的触变性却被忽视。触变性是流变学研究的重要部分。最初由Peterfi在1927年 [17] 提出的描述胶体溶液从流体条件到弹性固体凝胶的可逆变化 [18] 。在大量胶体体系中观察到类似的趋势后,文献中已广泛研究了胶体稀释悬浮液的触变性 [19] 。触变性的定义在科学界已达到普遍一致,即当流体施加到先前已经静止的样品时粘度随时间的连续降低,以及当流动时间随后的粘度恢复时停止 [20] 。因此,在本研究中,围绕丝胶凝胶的触变性展开了一系列研究。
2. 实验
2.1. 实验材料
家蚕蚕茧(苏豪生物技术有限公司),实验室用水均为去离子水。
2.2. 丝胶溶液的制备
本实验方法依据茧壳表层丝胶易溶于热水中,并且加压能加速这种溶解现象的原理 [1] 。取新鲜蚕茧,削口去除蚕蛹,去除茧衣与内衬,剪成1 cm2小块。称取一定质量的蚕茧,与去离子水按一定比例放入密闭容器中,将容器置于高温灭菌锅中加热一段时间后,弃去茧壳,所得溶液即为丝胶溶液。将丝胶溶液储存于80℃水浴中,测量溶液的质量分数后使用。
2.3. 溶液浓度随高温高压煮茧时间的关系
将洗净茧壳与去离子水按1:20置于高温高压灭菌锅中加热,以高温高压灭菌锅超压(0.15 MPa, 126℃)喷气时开始计时,分别测量0 min,1 min,5 min,10 min,15 min,20 min,25 min,30 min时所得丝胶溶液的浓度。
称取一定量的家蚕茧壳称重,其质量为m1,制备完丝胶溶液后,将茧壳在热的去离子水中反复洗净,于60℃烘干,称重,其质量为m2,按下式计算各组的脱胶率。
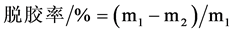 (1)
(1)
2.4. 凝胶临界浓度
将丝胶溶液配制成不同浓度0.1%,0.2%,0.3%,0.4%......1.0%,常温下静置10 h后,找出丝胶能够形成凝胶的临界浓度。
2.5. 粘度测试
选用AR2000型流变仪(美国)TA Instruments公司)测定按蚕茧和去离子水(w/v)比例1:10制备的丝胶溶液在25℃、37℃和60℃时的粘度变化。剪切速率是1000 rad/s,剪切时间1 min,取点80个。以第一次剪切作用开始计为0时刻,剪切后放置一段时间再次测试,共计间隔三段时间,分别为5 min,5 min,10 min。
2.6. 结构分析
丝胶溶液质量浓度为1.5%,在37℃条件下放置10 h后形成凝胶,将丝胶凝胶经过摇变后形成半凝胶状态,最后将半凝胶状态的丝胶再次静置于37℃条件下10 h使其再次凝胶。取溶液、凝胶、半凝胶及再凝胶四种状态下的丝胶在液氮中迅速冷冻固定,然后真空干燥备用。使用全自动X,PERT PRO MPD射线衍射仪(荷兰帕纳科公司),Cu Kα射线,X射线波长为λ = 1.5406,衍射角选取5˚~45˚间的衍射强度曲线。
丝胶溶液质量浓度为1.0%,在37℃条件下放置10 h后形成凝胶,将丝胶凝胶经过摇变后形成半凝胶状态,最后将半凝胶状态的丝胶再次静置于37℃条件下10 h使其再次凝胶。使用Aviv Model 410型圆二色光谱仪测定丝胶蛋白构象变化。吸取0.5 ml的混合溶液置于光径为0.1 mm的石英样品池中,在室温(25℃)下进行远紫外波段的光谱测试,扫描范围为190~250 nm。测量参数:波长步长为1.0 nm,扫描间隔0.5 nm,扫描速率50 nm/min,响应时间5 s。测量时对光源系统通氮气以除去空气和水汽,且所有样品的旋光度数值均扣除溶剂水自身所造成的背景值。
2.7. 截面形貌
丝胶溶液质量浓度为1.5%,在37℃条件下放置10 h后形成凝胶,将丝胶凝胶经过摇变后形成半凝胶状态,最后将半凝胶状态的丝胶再次静置于37℃条件下10 h使其再次凝胶。取溶液、凝胶、半凝胶及再凝胶四种状态下的丝胶在液氮中迅速冷冻固定,然后真空干燥备用。用切片器取一定体积的冻干样品,表面喷金90 s取出,采用日本Hitachi S-4800型扫描电子显微镜(SEM)观察凝胶的截面形貌。
2.8. 剪切作用前后丝胶的凝胶时间
静置丝胶溶液,将盛装丝胶溶液的容器倾斜45度后不发生流动,即凝胶形成,这段时间称为凝胶时间。丝胶溶液凝胶后,经摇变使其变为半凝胶状态,再次将容器静置,若容器倾斜45度不发生流动,这段时间称为再凝胶时间。取质量浓度为1.5%的丝胶溶液置于5 ml离心管中,将离心管分别放在4℃,25℃,37℃环境中,测其凝胶时间和再凝胶时间。取质量浓度为0.6%,0.9%,1.2%,1.5%,1.8%的丝胶溶液置于5 ml离心管中,将离心管分别放在37℃环境中,测其凝胶时间和再凝胶时间。
3. 结果与讨论
3.1. 丝胶凝胶
3.1.1. 丝胶溶液浓度和脱胶率随高温高压时间的关系
由图1中高温高压条件下不同煮茧时间所得丝胶溶液浓度所知,随着茧壳在水浴中时间越长,蚕丝表层的丝胶溶解于水中越多,丝胶溶液浓度越大,当高温高压灭菌锅内完成加压过程,即0 min时,丝胶溶液浓度已经达到约0.2%,当煮茧时间长达30 min时,丝胶溶液浓度已经高达1.349%;同时,从图中随煮茧时间而变化的脱胶率来看,时间越长,脱胶率越大,大概10 min左右时,脱胶率已经约高达23%,蚕茧表层丝胶大部分溶解于溶液中。将丝胶溶液浓度和脱胶率结合起来看,在高温高压条件下,10 min以前,变化速率较快,说明蚕茧表层丝胶更加容易溶解出来,10 min以后,速率变化变慢,且25 min和30 min时的结果基本一致,说明蚕茧表层丝胶溶出随着时间增长变得更加困难,这些结果也正好与文献 [7] 中一致。此种煮茧方式,蚕茧表层的大部分丝胶大约在30 min左右基本全部溶解出来。
3.1.2. 临界浓度
丝胶的凝胶化过程与氢键有关,而丝胶溶液的浓度会影响氢键的形成 [21] 。由图2所示,在室温(25℃)条件下,一定浓度的丝胶溶液经一段时间的静置后会形成凝胶。当丝胶溶液含固量低于0.3%时,即使经过长达10 h的静置时间,丝胶仍未凝胶;当丝胶溶液浓度高于0.3%时,丝胶溶液完成凝胶化转变。
3.2. 触变性
对丝胶凝胶施加一小段持续的剪切力作用后,丝胶凝胶变成半凝胶状态,可以发生流动,如图3所示。丝胶凝胶受到剪切力作用时,粘度急剧下降,待静置一段时间后,溶液再次形成凝胶,粘度恢复,重复施加剪切作用后,粘度又一次下降,丝胶凝胶表现出的即是正触变性。在这个过程中,丝胶凝胶经触变后,氢键遭到破坏,体系恢复的过程实质上也是断裂的氢键再次形成的过程,且体系恢复的快慢与温度有关。如图4所示,25℃和37℃环境中,丝胶蛋白从半凝胶状态完全转变成凝胶状态只需要5 min就能完成,而在60℃环境中这个过程需要10 min以上。丝胶触变后的再凝胶过程与温度的依赖性规律符合文献中的研究 [22] 。
3.3. 结构分析
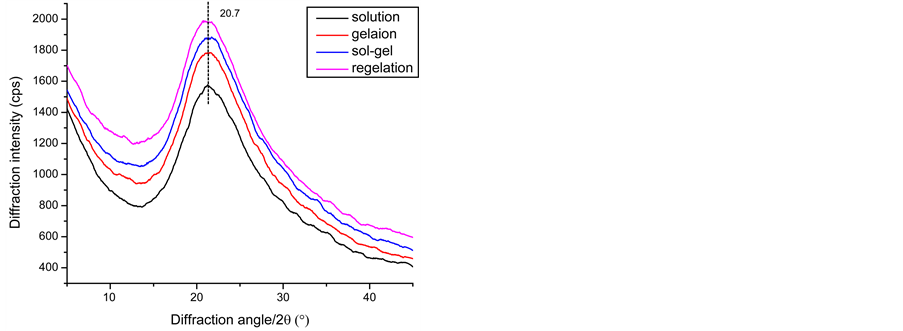
丝胶的二级结构由图5中XRD和CD所示。图5(a)中四种状态下的丝胶衍射峰是隆峰,且峰形基本一致,表明丝胶的聚集态结构以无规卷曲为主。如图5(b)中圆二色谱分子构象显示,丝胶在溶液状态无明显峰形,分子构象基本呈无规卷曲状态;凝胶状态在197 nm出现正峰和217 nm附近出现负峰,表明丝胶分子构象从无规卷曲变成β-折叠结构 [23] ;半凝胶状态和再凝胶状态与凝胶状态分子构象变化不大。由丝胶的结构分析知,丝胶溶液形成凝胶分子构象从无规卷曲转变成β折叠构象,但聚集态结构上呈非晶结构,且触变剪切作用并不改变丝胶分子的聚集态结构,也从侧面验证了前人提出的丝胶凝胶的形成与氢键 [21] 有关。

Figure 1. Mass concentration and degumming percentage of sericin
图1. 高温高压条件下丝胶的溶液含固率和脱胶率

 (a) (b)
(a) (b)
Figure 3. (a) Sol-gel system before shear; (b) Sol-gel system after shear
图3. (a) 剪切作用前的丝胶凝胶;(b) 剪切作用后的丝胶凝胶
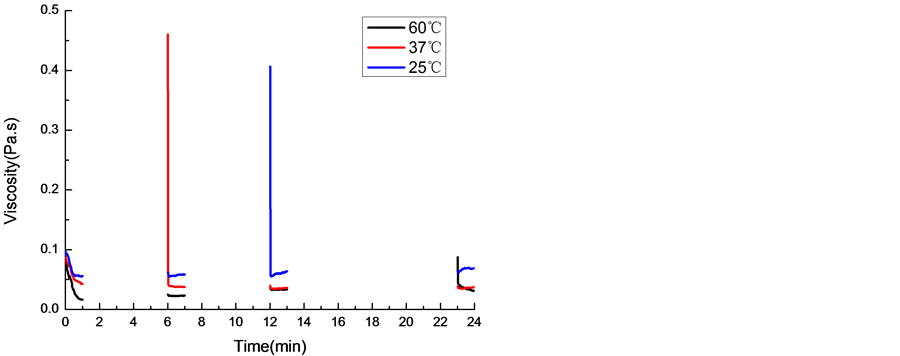
Figure 4. Viscosity of gel under a discrete shearing forces. The sericin solution boiled at a ratio of 1:10, then formed gel at room temperature
图4. 不同温度下的丝胶溶液收到剪切作用后的粘度变化
 (a) (b)
(a) (b)
Figure 5. (a) XRD images of sericin whose mass concentration is 1.5% in 4 different state at 37˚C; (b) CD images of sericin whose mass concentration is 0.93% in 4 different state at 37˚C
图5. (a) 1.5%的丝胶在四种状态下的XRD;(b) 0.93%的丝胶在四种状态下的CD
3.4. 截面形貌
如图6所示,在触变过程中四种状态下丝胶截面形貌。图A是丝胶溶液,有大量的微球结构和线型结构。图B是凝胶结构,微球结构逐渐聚集成线型结构,线型结构逐渐聚集成片状结构,结构中以片状结构为主,少量微球、线型结构,说明凝胶化进程中形成了较为稳定的网状结构。图C是丝胶凝胶经剪切后的半凝胶状态,部分片状结构和线型结构被破坏,但仍以片状结构为主,此过程中网状结构被破坏。图D是半凝胶状态的丝胶经再次静置后形成的凝胶,与图C半凝胶状态相比,聚集效应明显,微球逐渐聚集成线型,线型逐渐聚集成较小的片状,破坏的片状结构再次聚集成片;与图B中凝胶状态相比,结构更分散,片状结构没有图B完整,说明丝胶凝胶的网状结构遭到破坏以后,即使能再次聚集形成网状结构,但难以恢复到破坏前的稳定状态。丝胶的截面形貌分析也说明丝胶凝胶的形成是氢键引起的分子聚集,触变剪切作用只是破坏这种网状结构部分的连接点,但丝胶分子仍以稍小的聚集成片的形式存在,与结构分析结果相符。
3.5. 剪切作用后的凝胶时间和再凝胶时间
由图7可知,丝胶溶液的浓度对丝胶凝胶化进程影响很大。总体上来讲,随着丝胶溶液浓度的增大,凝胶速度较快,凝胶时间逐渐缩短。此结果与文献 [24] 中结果相同,1.8%的丝胶溶液凝胶时间相比于0.6%的丝胶溶液凝胶时间缩短了将近4倍。将丝胶凝胶切稀后,不同含固量的丝胶凝胶再凝胶时间差异明显。
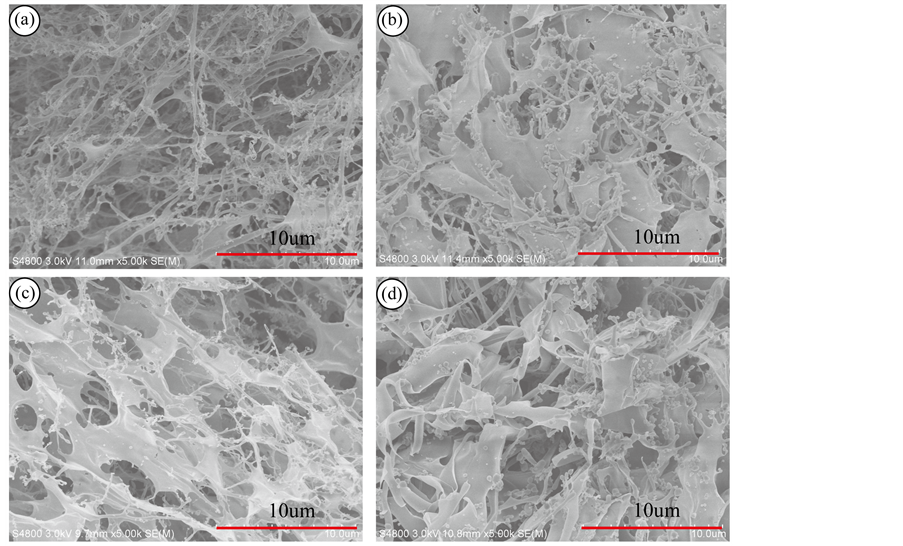
Figure 6. Cross section morphology of sericin whose mass concentration is 1.5% at 37˚C: (a) Liquid; (b) Gel; (c) Sol-gel; (d) Re-gel
图6. 在37℃条件下,1.5%的丝胶在不同状态下的SEM图片:(a) 溶液状态;(b) 凝胶状态;(c) 半凝胶状态;(d) 再凝胶状态
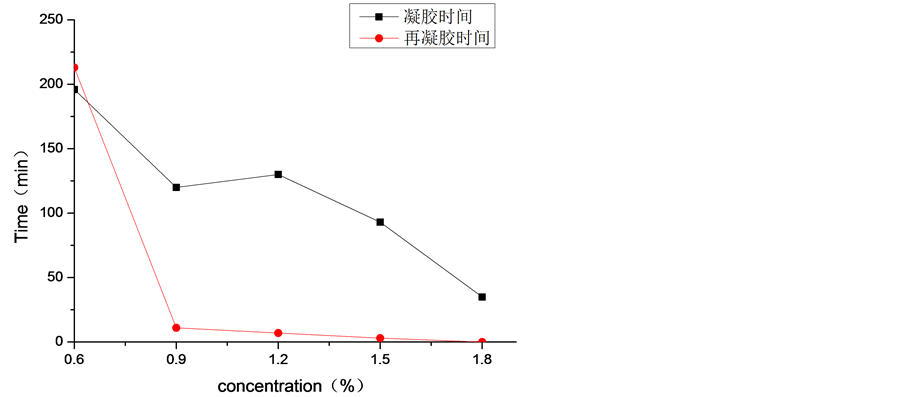
Figure 7. Gelation and regelation time of sericin solution with different concentration at 37˚C
图7. 37℃下不同浓度的丝胶溶液的凝胶时间和再凝胶时间
对于浓度比较低的0.6%的丝胶凝胶,再凝胶时间长达3.5 h,长于凝胶时间。更加值得关注的是,含固量高于0.9%以上的丝胶凝胶在几分钟之内完成了再凝胶过程,远远低于0.6%的丝胶凝胶再凝胶时间。导致这种结果的原因是,丝胶中含有极性侧链的氨基酸相互之间形成氢键,分子间的空隙较大,结合较弱,这种现象越明显 [21] [25] ;当氢键形成的网状结构遭到剪切力作用后,结合力越弱的很容易就被破坏,氢键再次形成难度大,丝胶浓度越大,氢键再次形成更加容易。
4. 结论
丝胶溶液的临界凝胶浓度为3 mg/ml,高于此浓度则容易凝胶化。丝胶凝胶后具有触变性,在剪切应力下具有流动性,消除剪切应力后又会凝胶化,为一种可逆凝胶。丝胶凝胶在剪切应力作用下,凝胶内部的氢键网络被破坏,从而造成体系粘度下降并呈现出半凝胶状态;停止剪切作用静置一段时间后,氢键重建,体系粘度恢复。剪切作用不破坏丝胶大分子的聚集态结构。丝胶凝胶经触变后体系恢复的快慢与丝胶含固量和温度有关。该可逆凝胶预计可以用于可注射生物材料。
基金项目
国家自然科学基金项目(51373114);江苏省高校自然科学研究重大项目资助(15KJA540001)。
*通讯作者。