1. 研究背景
结肠癌在我国的发病率日渐升高:2012年我国结肠癌发病率位居常见恶性肿瘤的第三位,死亡率约14.23/10万,居于常见恶性肿瘤的第五位 [1] 。目前除传统手术、化疗和放疗等常规治疗外,免疫治疗在多种实体肿瘤的治疗中已获得令人瞩目的效果,尤其是抗PD-1、抗CTLA-4等免疫制剂的问世更令人鼓舞。但由于目前对肿瘤免疫微环境的认识有限,导致免疫治疗效果存在较大的个体差异,也限制了其临床应用。
目前认为肿瘤细胞可通过调控蛋白表达对自身周围微环境进行“改造”,包括影响免疫细胞的数量和活性。如肿瘤细胞能通过下调HLA抗原和增加MICA/MICB蛋白的表达,削弱NK细胞的攻击性 [2] ;或通过上调PD-1配体(PD-L1)表达以促进浸润淋巴细胞凋亡、削弱抗肿瘤免疫应答 [3] 。我们的前期研究也发现,结肠癌表达的gal-3能在巨噬细胞的参与下增强肿瘤细胞的侵袭能力。这些结果均提示结肠癌可通过调控某些蛋白而影响微环境中的免疫细胞和构建有利于其生长的环境。辅助性T细胞17 (T helper cell-17,Th17)和调节性T细胞(T regulatory cell,Treg)是两种与肿瘤发生、发展关系密切的免疫细胞。Th17细胞主要表达白介素-17 (interleukin-17,IL-17),可诱导局部炎症和新生血管形成。Treg细胞属于免疫抑制细胞,主要表达Foxp3蛋白,可通过表达IL-10等细胞因子调控细胞免疫应答。在多种实体瘤中可观察到这两种细胞有不同程度的聚集,但其影响其聚集和分布的因素仍未清楚。
因此,深入了解肿瘤微环境与免疫细胞的相互关系,有助进一步研究肿瘤的免疫应答机制,为寻找新的抗肿瘤治疗和靶点提供理论依据。基于此,本研究拟通过分析结肠癌组织中gal-3蛋白表达与微环境中Foxp3+、IL-17+淋巴细胞的相关性,初步探讨结肠癌相关蛋白表达对微环境中免疫细胞的影响。
2. 研究方法
2.1. 实验材料
收集2011~2012年在广州医科大学附属肿瘤医院进行手术切除的结肠癌组织,包括50例肿瘤组织及10例正常结肠粘膜组织。所有组织均经过甲醛固定和石蜡包埋,每例标本切取4 μm白片10张,其中1张进行HE染色以确定肿瘤组织,剩余切片进行免疫组织化学染色。病例组织入选标准:组织来源于18~70岁的结肠癌患者,性别不限,术前均未进行新辅助放化疗、生物治疗、中医药治疗等其它抗肿瘤治疗;排除自身免疫疾病患者及接受免疫抑制药物治疗者;术后报告淋巴结数目不少于12枚,以获得准确临床分期;术后病理确诊为结肠腺癌,并有完整的临床病理资料。
2.2. 免疫组化染色
所有收集的组织均经过HE染色证实为结肠腺癌组织或正常结肠粘膜。免疫组化染色采用二步法进行 [4] :组织切片脱蜡、水化、修复抗原,PBS洗涤后加入3%过氧化氢阻断内源性过氧化物酶,反应10分钟,稀释一抗孵育4℃过夜,PBS洗涤3次后二抗孵育室温 30分钟,二氨基联苯氨(DAB)染色。结果判断由2名病理科医师双盲阅片。主要抗体包括:兔抗人IL-17A多克隆抗体(Abcam公司,ab136668)、鼠抗人FOXP3单克隆抗体(ab22510)、鼠抗人galectin-3单克隆抗体(ab2785)。
2.3. 结果评定
所有病理染色切片均有两名病理科医师采用双盲法分别进行阅片和记录结果。切片先置于低倍镜(X 40)下观察,选取癌巢和间质组织中浸润淋巴细胞密度最高的区域,然后转换高倍镜(X 400)随机选取5个不同视野进行阳性染色细胞计数。Foxp3+、IL-17+淋巴细胞应为细胞浆被特异性着色为淡黄色至棕褐色。连续计数每100个淋巴细胞中阳性细胞数,阳性细胞数<10%为阴性,阳性细胞数 > 10%为阳性。Gal-3阳性染色评分根据既往文献方法进行 [4] :Gal-3在肿瘤组织中定位于胞浆,按细胞着色程度评分为:棕褐色3分,棕黄色2分,淡黄色1分,无着色为0分。按着色细胞的数量评分:一个视野内着色细胞大于75%为4分,51%~75% 为3分,11%~50%为2分,小于10%为1分,阴性为0分。最后两者相加,0~3分为阴性表达,4~6分为阳性表达。
2.4. 统计分析
统计分析采用SPSS 13.0软件,目的蛋白表达情况与临床病理参数的关系采用χ2检验或Fisher确切概率法;各目的蛋白表达的相关性分析采用spearman相关分析。当P < 0.05时,认为具有统计学意义。
3. 结果
3.1. Galectin-3、Foxp3和IL-17A在结肠癌组织中的表达
通过镜下观察免疫组化结果,发现gal-3蛋白(棕色染色)主要表达于肿瘤组织的细胞质,在癌旁正常细胞中的表达主要定位于细胞核(见图1)。在50例结肠癌肿瘤组织中,gal-3表达阳性率为66% (33/50),正常结肠上皮组织中仅1例gal-3为阳性表达(阳性率10%,1/10),提示肿瘤组织与正常结肠上皮组织中gal-3的表达有明显差异(P = 0.001)。在I-II期结肠癌组织中gal-3阳性率为46% (12/26),而在III~IV期结肠癌组织中表达阳性率为87.5% (21/24),两组gal-3的表达水平也有统计学差异(P = 0.003)。但相关性分析提示肿瘤组织gal-3的表达与年龄、性别临床因素无明显关系(见表1)。
Foxp3蛋白主要表达在结肠癌间质组织中的淋巴细胞,一般认为是调节性T细胞(Treg)的主要标记蛋白。免疫组化染色显示Foxp3蛋白主要定位于淋巴细胞的细胞核,这种Foxp3+细胞主要分布于肿瘤间质中,少部分浸润于肿瘤组织中(见图2)。统计结果显示,在结肠癌间质中Foxp3+细胞阳性率高于正常上皮组织的阳性率(54% vs 10%,P = 0.005);而且Foxp3+细胞的阳性率也与肿瘤TNM分期显著相关(P = 0.005),但其阳性率与肿瘤大小、部位、浸润深度、分化程度、性别、年龄等其他临床病理参数的差异均无统计学意义(见表1)。
此外,染色结果也发现部分结肠癌细胞的胞浆中有Foxp3阳性染色,在细胞核中则无明显染色。结

Table 1. Correlation between expression of gal-3, Foxp3, IL-17A and clinicopathologic characteristic in colon cancer tissues
表1. 结肠癌组织gal-3、Foxp3和IL-17A表达与临床病理参数的关系
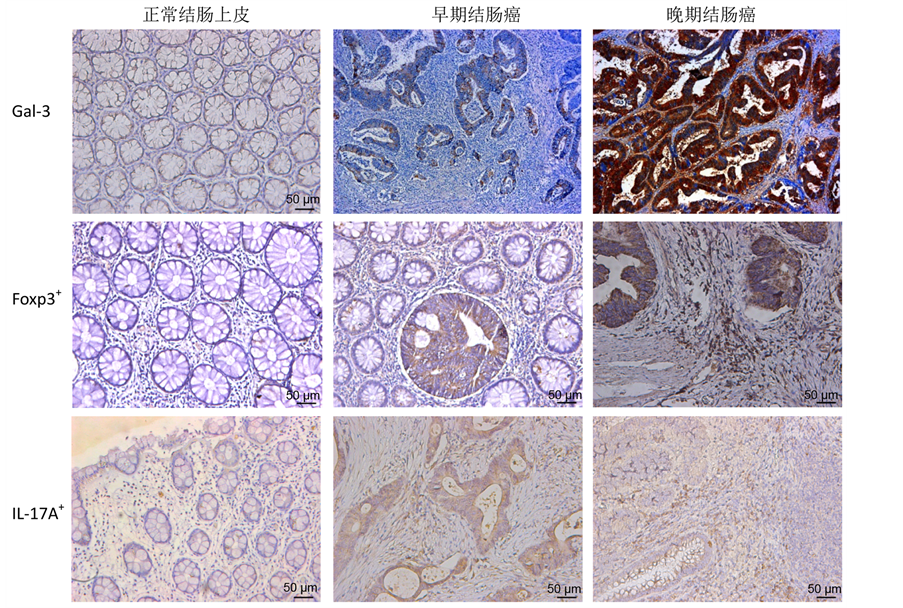
Figure 1. Different expressions of galectin-3 (gal-3), Foxp3+ lymphocytes and IL-17A+ lymphocytes in normal colon epithelium, early-stage and late-stage colon cancer
图1. Galectin-3 (gal-3) 蛋白、Foxp3+淋巴细胞和IL-17A+淋巴细胞在正常结肠上皮、早期和晚期结肠癌组织中的表达差异

Figure 2. Expressions and distributions of Foxp3+ lymphocytes and IL-17A+ lymphocytes in colon cancer tissues
图2. Foxp3+淋巴细胞和IL-17A+淋巴细胞在结肠癌组织中的表达和分布
肠癌细胞Fopx3的阳性率(60%)也显著高于正常肠粘膜上皮细胞(10%) (P = 0.001)。但这种Fopx3+肿瘤细胞比例在早期(I-II期)与中晚期(III-IV期)病例之间无明显差异(54% vs 65%,P = 0.565),也与性别、年龄等其他临床-病理参数无明显相关性(见表1)。
IL-17A是Th17淋巴细胞的主要标记蛋白。本研究结果发现在结肠癌组织中IL-17A+细胞主要为肿瘤间质中的淋巴细胞(见图1、图2)。这类IL-17A+淋巴细胞在所有结肠癌组织中的阳性率为76%,明显高于正常上皮组织中的阳性表达率(76% vs 20%,P = 0.001)。在中晚期肿瘤组织中的阳性率也高达83%,明显高于早期肿瘤组织(83% vs 69%,P = 0.035)。但肿瘤组织中IL-17A+细胞的比例与性别、年龄、肿瘤浸润深度等临床-病理参数均无相关性(见表1)。
3.2. 结肠癌组织中gal-3、Foxp3、IL-17A的表达相关性
虽然以上结果表明在结肠癌组织中gal-3蛋白、Foxp3+和IL-17A+淋巴细胞的表达率明星高于正常结肠上皮组织,但由于gal-3表达可影响微环境中T淋巴细胞的数量和分布,因此我们仍进一步分析gal-3与Foxp3、IL-17A的表达相关性。分析结果表明,结肠癌组织的gal-3表达水平与肿瘤间质中Foxp3+细胞的比例呈正相关(r = 0.608,P < 0.001),即肿瘤细胞表达gal-3越高,间质组织中浸润的Fopx3+细胞越多。此外,肿瘤组织gal-3蛋白水平也与肿瘤间质中IL-17A+细胞的阳性率正相关(r = 0.289,P = 0.042),提示肿瘤组织gal-3表达水平越高,间质中浸润IL-17A+细胞越多。但局部组织中Foxp3+细胞和IL-17A+细胞之间的比例和分布无明显相关性(见表2)。
4. 讨论
结肠癌的发生与结肠局部慢性炎症关系密切,而肿瘤形成后产生的蛋白和细胞因子也对局部微环境中的免疫因素有重要影响。目前越来越多研究证实,免疫应答的失衡与肿瘤进展、临床预后密切相关 [5] 。虽然结肠癌在发生、发展过程中对机体免疫系统起着重要作用,但肿瘤产生的蛋白如何影响局部微环境中的免疫细胞仍未清楚。本文通过免疫组化方式检测了结肠癌组织中的gal-3蛋白与Foxp3+细胞和IL-17A+细胞的关系,发现三者在肿瘤组织中的表达水平均显著高于正常对照组织,且Foxp3+细胞和IL-17A+细胞数量与肿瘤gal-3表达水平密切相关。
Gal-3是凝集素家族中半乳糖凝素的成员,是一种多功能蛋白,其功能涉及细胞间的粘附、新生血管形成、细胞凋亡调控和免疫识别等。Gal-3在多种实体肿瘤中呈高表达,参与肿瘤细胞的生长、抗凋亡和促进转移,并可通过与肿瘤浸润淋巴细胞的相互作用,调控淋巴细胞的活性。已证实结肠癌组织gal-3的表达水平与肿瘤浸润深度、淋巴结转移、临床分期密切相关 [6] [7] ;我们的前期研究也发现gal-3是影响结肠癌患者预后的独立因素。但目前关于gal-3与肿瘤免疫应答的研究不多,曾有研究发现gal-3可调节人白血病Jurkat T细胞的生长和凋亡 [8] 。肿瘤产生的gal-3在局部微环境中有高浓度聚集,这种局部高浓度的gal-3能与肿瘤反应性T细胞表面的免疫突触结合,刺激T细胞分泌细胞因子,促进其凋亡,

Table 2. Correlation among gal-3, IL-17A and Foxp3 expressions in colon cancer tissues
表2. 结肠癌组织中gal-3、IL-17A、Foxp3表达的相互关系
减弱抗肿瘤免疫应答 [9] 。
关于结肠癌组织中IL-17+淋巴细胞及Foxp3+淋巴细胞增多的机制尚未明确。由于IL-17主要来源于Th17细胞,而肿瘤周围浸润间质中Foxp3主要表达于Treg,所以这两种蛋白在局部组织中的表达变化,实际上基本反映了Th17细胞和Treg细胞在肿瘤微环境中浸润情况。既往文献报道Foxp3+淋巴细胞在肿瘤组织的聚集与患者的不良预后有关 [10] [11] 。本研究亦观察到Foxp3+淋巴细胞在肿瘤周围间质中的表达明显高于正常肠粘膜,且与临床分期相关。既往研究也发现结肠癌组织中有聚集增多的IL-17+淋巴细胞,而且这类IL-17+细胞的聚集密度与患者疾病进展、临床预后呈负相关 [11] 。尽管如此,目前关于IL-17+淋巴细胞的作用尚有争论:虽有不少研究发现肿瘤组织的IL-17+淋巴细胞明显增多,而且动物模型也证实Th17细胞是促使慢性肠炎演变为结肠癌的重要因素 [12] ,但最新观点也认为IL-17+淋巴细胞可通过CCL5/CCL20、IL-8招募CD8+毒性T细胞和中性粒细胞进入肿瘤组织,并直接影响针对肿瘤的免疫反应 [13] ,而IL-17+淋巴细胞并非直接影响因素。我们的研究结果显提示在正常肠粘膜、早期肿瘤和晚期肿瘤组织中,IL-17A+淋巴细胞的比例也是逐渐升高。这提示在结肠癌发展过程中肿瘤局部聚集的FOXP3+淋巴细胞和IL-17A+淋巴细胞逐渐增多,可能通过免疫抑制和诱导新生血管形成,参与肿瘤细胞的生长和侵袭。
关于肿瘤细胞如何通过改变微环境招募和维持肿瘤浸润淋巴细胞(tumor infiltrating lymphocytes, TIL)的机制仍未清楚。有观点认为肿瘤产生的IL-6对TIL的比例起重要作用 [14] :初始CD4+T细胞在TGF-ß诱导下可表达Foxp3,分化成为Treg细胞;但TGF-β和IL-6共同刺激下可使初始CD4+T中Foxp3的表达被抑制,转向启动RORγt的表达,使CD4+T细胞向Th17细胞方向分化。因此,IL-6在决定CD4+T细胞向Treg/Th17细胞的分化方向中起关键作用 [15] 。我们的前期研究也发现,随着肿瘤进展,肝癌组织中Foxp3+淋巴细胞和IL-17+淋巴细胞比例明显增多,但这种趋势与局部IL-6表达水平无明显相关性。因此我们将研究目标转移至与结肠癌密切相关的gal-3蛋白。半乳糖凝集素家族成员与多种疾病的免疫调节有关:gal-1可诱导胸腺细胞及外周活化的T细胞的凋亡,在小鼠体内可减少Th1细胞中IFN-γ的分泌,抑制肠道炎症的发生 [16] [17] ;重组的gal-1也可通过诱导CD4+调节性T细胞,进而抑制Th1介导的自身免疫性视网膜病 [18] [19] 。Gal-2和gal-9也可促进活化的T细胞发生凋亡 [19] 。在炎症性肠病中,gal-4可刺激肠粘膜的CD4+T细胞分泌IL-6,加剧肠道炎症 [20] 。
本研究结果显示肿瘤组织gal-3的表达与肿瘤周围间质中Foxp3+淋巴细胞和IL-17A+淋巴细胞的比例呈正相关,提示在结肠癌进展过程中,肿瘤表达的gal-3可能影响周围微环境中淋巴细胞的浸润和分布,对淋巴细胞有重要的介导作用。通过诱导如Foxp3+、IL-17A+淋巴细胞的浸润,形成免疫抑制状态和诱导新生血管形成,肿瘤可构建适合自身生长的微环境,促进肿瘤进展。然而,由于样本量偏少,gal-3与Foxp3+淋巴细胞、IL-17A+淋巴细胞之间的关系及与结肠癌进展的相关性仍需通过大样本分析进一步验证;且对于gal-3如何调控TIL数量和比例的具体分子机制亦有待深入研究。Gal-3能否影响TIL的PD-1/CTLA-4表达将是一个有趣的问题,这可为未来免疫靶向治疗提供更多的思路和参考。
5. 结论
综上所述,在结肠癌发展的过程中,随着肿瘤局部gal-3蛋白表达升高,肿瘤组织中浸润的Foxp3+淋巴细胞和IL-17A+淋巴细胞也明显增多,提示结肠癌细胞可能通过提高局部gal-3的表达水平,招募和维持更多的肿瘤浸润性淋巴细胞参与肿瘤的进展。
基金项目
广东高校优秀青年创新人才培养计划(自然科学) (39GZMU-10)、广州医科大学博士启动项目(2011A32)。
NOTES
*通讯作者。