1. 引言
众所周知,吲哚作为一类具有独特结构和生物活性的化合物,在药物、功能材料和香料等领域应用广泛 [1] [2] [3] [4] [5] 。β-吲哚酮化合物在吲哚化学的研究中具有重要地位,迈克尔加成反应是合成该类化合物的有效途径之一 [6] [7] [8] [9] [10] 。
近年来,离子液体因具有低毒、低挥发性、良好的溶解性能和可循环使用等优点被广泛关注 [11] [12] [13] [14] 。离子液体作为反应溶剂、催化剂或促进剂应用于有机合成反应,可以高效、高选择性且条件温和的实现化合物之间的转化,符合绿色化学的理念 [15] [16] 。基于本课题组在离子液体领域的研究基础 [17] [18] ,本文采用Brønsted酸性功能化噻唑硫酮离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]催化吲哚与α,β-不饱和酮发生迈克尔加成反应,以82%~99%的产率得到了一系列β-吲哚酮化合物。本方法优点在于底物普适性好、产物产率高,催化剂可以循环使用3次以上催化活性无明显下降。
2. 实验部分
2.1. 仪器与试剂
瑞士Buchi B-540型熔点仪;德国Bruker Equinox 55红外光谱仪(KBr压片);美国Varian inova-400型核磁共振仪(TMS为内标,CDCl3、D2O或DMSO-d6为溶剂);美国HP1100液相色谱质谱仪。所有试剂均为市售分析纯,用前未经处理直接使用。
2.2. Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]合成
Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]的合成参照文献 [19]
2.3. α,β-不饱和酮的合成
α,β-不饱和酮的合成参照文献 [20] 。
2.4. β-吲哚酮化合物3a-3z的合成及结构分析
吲哚化合物(1 mmol)、α,β-不饱和酮化合物(1 mmol)、Brønsted酸性离子液体催化剂[ThiN(CH2)4SO3][p-CH3PhSO3] (10 mol%)和乙腈(5 mL)在80℃下磁力搅拌反应5 h。反应结束后,待反应混合物冷至室温,加入碎冰搅拌至碎冰溶化并析出固体,过滤,用蒸馏水洗涤固体得到粗产物。粗产物经硅胶柱层析分离纯化得到目标产物纯品,反应式如式1所示。所得化合物结构经1H NMR,13C NMR,IR和MS确征。
未知化合物的结构表征如下:
化合物3h:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 7.99 (s, 1H), 7.90-7.92 (m, 2H), 7.92 (d, J = 7.6 Hz, 2H), 7.46-7.52 (m, 1H), 7.39 (d, J = 7.6 Hz, 1H), 7.27-7.32 (m, 1H), 7.14 (t, J = 8.0 Hz, 1H), 6.88-7.03 (m, 6H), 5.03 (t, J = 8.0 Hz, 1H), 3.84 (s, 3H), 3.60-3.76 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.91, 163.47, 162.53, 160.10, 140.01, 139.98, 136.63, 130.37, 130.12, 129.29, 129.21, 126.48, 122.23, 121.29, 119.47, 119.44, 119.33, 115.24, 115.03, 113.73, 111.15, 76.70, 55.47, 44.80, 37.64; IR (KBr), υmax/cm-1: 3390, 3048, 2908, 2838, 1659, 1598, 1507, 1419, 1355, 1253, 1218, 1180, 1020, 980, 837, 742, 591, 534; ESI-MS: m/z (%) = 396 (100) [M+Na] +.
化合物3j:土黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 7.88 (d, J = 12.0 Hz, 3H), 6.86-7.48 (m, 10H), 4.98 (t, J = 8.0 Hz, 1H), 4.04-4.09 (m, 2H), 3.59-3.77 (m, 2H), 2.17 (s, 6H ), 1.40 (t, J = 4.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 197.16, 162.76, 141.82, 136.59, 136.38, 134.27, 130.38, 130.10, 129.59, 129.17, 126.74, 124.97, 121.99, 121.34, 119.73, 119.61, 119.28, 114.08, 111.01, 76.69, 63.71, 44.97, 37.89, 19.89, 19.32, 14.66; IR (KBr), υmax/cm-1: 3335, 3041, 2982, 2936, 2889, 1649, 1592, 1420, 1357, 1259, 1171, 1041, 816, 741, 610; ESI-MS: m/z (%) = 420 (100) [M+Na] +.
化合物3k:紫红色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 8.05 (s, 1H), 7.87-7.91 (m, 2H), 6.86-7.50 (m, 10H), 5.01 (s, 1H), 4.04-4.10 (m, 2H), 3.56-3.75 (m, 2H), 1.41 (t, J = 8.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.46, 163.02, 158.75, 156.30, 141.95, 141.92, 136.63, 132.64, 130.37, 129.77, 128.45, 128.37, 126.33, 122.36, 121.31, 119.59, 119.28, 118.67, 116.29, 116.07, 114.22, 111.24, 108.93, 108.72, 76.71, 63.79, 44.57, 37.34, 14.65; IR (KBr), υmax/cm-1: 3331, 3058, 2983, 2935, 2895, 1645, 1598, 1493, 1334, 1246, 1172, 1042, 980, 813, 738, 612; ESI-MS: m/z (%) = 490 (100) [M+Na] +.
化合物3l:淡黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm): 7.92-7.99 (m, 2H), 6.80-7.39 (m, 9H), 5.46 (t, J = 8.0 Hz, 1H), 4.06-4.11 (m, 2H), 3.72-3.84 (m, 1H), 3.53-3.76 (m, 2H), 1.55 (s, 1H), 1.41 (t, J = 8.0 Hz, 3H);
13C
NMR (CDCl3, 100 MHz): δ (ppm) : 196.56, 141.28, 137.40, 137.29, 136.69, 136.58, 134.57, 133.14, 130.17, 129.69, 129.07, 127.10, 126.87, 126.14, 124.83, 121.72, 119.48, 118.90, 117.74, 109.31, 109.21, 76.69, 45.42, 37.78, 32.69, 19.91, 19.34; IR (KBr), υmax/cm-1: 3384, 3059, 2980, 1652, 1598, 1484, 1340, 1248, 1173, 1041, 907, 844, 742, 640; ESI-MS: m/z (%) = 444 (100) [M+Na] +.
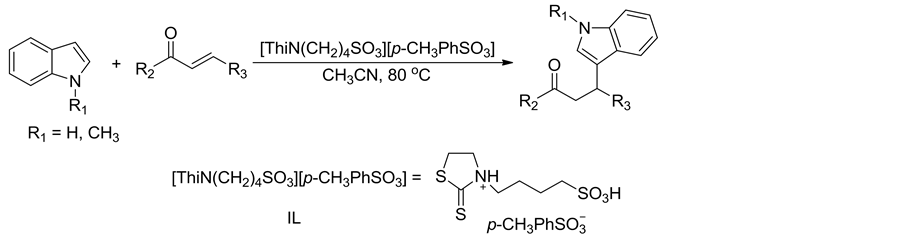
Scheme 1. Synthesis of β-indolone compounds
式1. β-吲哚酮化合物的合成
化合物3m:褐色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.94 (s, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.00-7.06 (m, 9H), 4.98 (t, J = 8.0 Hz, 1H), 3.61-3.75 (m, 2H), 2.18 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.57, 196.02, 141.09, 137.43, 136.68, 136.57, 134.62, 133.15, 130.61, 130.15, 129.69, 129.06, 127.09, 126.51, 124.88, 122.20, 121.28, 119.46, 111.10, 58.49, 45.27, 37.89, 19.90, 19.33; IR (KBr), υmax/cm-1: 3393, 3040, 2922, 1677, 1580, 1453, 1387, 1276, 1190, 1026, 880, 802, 741, 523; ESI-MS: m/z (%) = 444 (45) [M+Na] +.
化合物3o:淡粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.91-7.94 (m, 2H), 7.16-7.57 (m, 7H), 7.24 (t, J = 3.6 Hz, 1H), 6.99-7.03 (m, 1H), 6.90-6.95 (m, 2H), 6.83 (s, 1H), 5.02 (t, J = 8.0 Hz, 1H), 3.75 (d, J = 9.6 Hz, 2H), 3.74 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.57, 141.09, 137.43, 136.68, 136.57, 134.62, 133.15, 130.61, 130.15, 129.69, 129.06, 127.09, 126.51, 124.88, 122.20, 121.28, 119.46, 119.27, 111.10, 76.69, 45.27, 37.89, 19.90, 19.33; IR (KBr), υmax/cm-1: 3058, 2878, 1673, 1569, 1505, 1448, 1308, 1223, 1155, 1012, 976, 830, 742, 690; ESI-MS: m/z (%) = 380 (100) [M+Na] +.
化合物3q:草绿色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.92 (d, J = 7.6 Hz, 2H), 7.46-7.52 (m, 4H), 7.19-7.32 (m, 5H), 7.11-7.15 (m, 1H), 7.01 (t, J = 1.6 Hz, 1H), 6.84 (s, 1H), 5.12-5.16 (m, 1H), 3.74 (s, 3H), 3.32-3.3.52 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 188.35, 164.42, 163.01, 161.89, 138.54, 138.53, 136.34, 136.23, 130.92, 130.50, 129.86, 129.83, 129.07, 128.98, 124.37, 124.34, 117.69, 117.44, 114.81, 114.59, 114.31, 76.69, 63.80, 14.67.; IR (KBr), υmax/cm-1: 3056, 2909, 2823, 1673, 1592, 1471, 1371, 1237, 1196, 1071, 998, 881, 737, 686; ESI-MS: m/z (%) = 442 (100) [M+Na] +.
化合物3t:土黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.75-7.79 (m, 2H), 7.54-7.57 (m, 2H), 7.43 (d, J = 8.0 Hz, 1H), 7.14-7.35 (m, 7H), 6.99-7.03 (m, 1H), 6.81 (s, 1H), 5.02 (t, J = 8.0 Hz, 1H), 3.73-3.79 (m, 2H), 3.72 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 197.54, 144.10, 137.30, 135.80, 131.83, 129.61, 128.45, 128.12, 127.72, 126.87, 126.33, 126.18, 121.73, 119.50, 118.89, 117.58, 109.30, 109.22, 45.22, 38.17, 32.71; IR (KBr), υmax/cm-1: 3054, 2885, 1679, 1580, 1481, 1397, 1243, 1194, 1069, 972, 828, 740, 697; ESI-MS: m/z (%) = 442 (75) [M+Na] +.
化合物3u:粉色粉末;1H NMR (CDCl3, 400 MHz, TMS): δ (ppm) : 7.83-7.85 (m, 2H), 7.16-7.43 (m, 7H), 7.01 (t, J = 7.6 Hz, 1H), 6.77-6.80 (m, 3H), 4.97 (t, J = 8.0 Hz, 1H), 3.74 (s, 3H), 3.71 (s, 3H), 3.62-3.70 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 188.07, 146.42, 140.47, 137.96, 137.35, 137.09, 133.19, 132.12, 130.68, 130.41, 130.32, 129.77, 127.48, 126.38, 119.66, 76.68, 19.95, 19.77; IR (KBr), υmax/cm-1: 3050, 2934, 2834, 1677, 1585, 1508, 1466, 1250, 1173, 1092, 1035, 1009, 976, 829, 802, 740; 7.88-7.92 (d, 2H, CH), 7.18-7.21 (d, 2H, CH), 7.04-7.06 (d, 2H, CH); ESI-MS: m/z (%) = 426 (98) [M+Na] +.
化合物3v:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.75 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 8.8 Hz, 2H), 7.46 (d, J = 8.0 Hz, 1H), 6.99-7.25 (m, 7H), 6.79 (s, 1H), 3.62-3.66 (m, 2H), 3.76 (s, 3H), 2.18 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 197.64, 141.50, 137.27, 136.47, 135.86, 134.42, 131.77, 129.62, 129.08, 128.00, 126.92, 126.12, 124.82, 121.63, 119.50, 118.81, 117.89, 109.15, 77.30, 77.19, 76.99, 76.67, 45.36, 37.71, 32.67, 19.89, 19.32; IR (KBr), υmax/cm-1: 3044, 2915, 1677, 1580, 1478, 1377, 1248, 1197, 1069, 1004, 975, 816, 744; ESI-MS: m/z (%) = 468 (100) [M+Na] +.
化合物3w:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.92-7.96 (m, 2H), 6.80-7.41 (m, 10H), 5.46 (t, J = 8.0 Hz, 1H), 4.09 (dd, 2H, J = 12.0, 7.2 Hz), 3.73-3.75 (m, 1H), 3.72 (s, 3H), 3.54-3.60 (m, 1H), 1.45 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.30, 162.91, 162.01, 159.54, 137.87, 137.83, 137.29, 133.97, 133.87, 130.40, 129.89, 129.81, 129.69, 126.92, 126.55, 121.79, 119.42, 118.94, 116.94, 116.70, 116.16, 114.14, 113.93, 109.19, 76.66, 63.73, 43.95, 34.36, 32.75, 14.65; IR (KBr), υmax/cm-1: 3051, 2981, 2902, 1668, 1599, 1483, 1315, 1244, 1177, 1040, 904, 846, 745, 600; ESI-MS: m/z (%) = 458 (100) [M+Na] +.
化合物3x:砖红色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.89-7.92 (m, 2H), 7.46 (d, J = 8.0 Hz, 1H), 6.86-7.25 (m, 8H), 6.80 (s, 1H), 4.97 (t, J = 8.0 Hz, 1H), 4.07 (d, J = 7.2 Hz, 2H), 3.61-3.75 (m, 5H), 2.18 (s, 6H), 1.42 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz):δ (ppm) : 197.08, 162.73, 141.97, 137.28, 136.37, 134.21, 130.37, 130.08, 129.58, 129.16, 127.06, 126.16, 124.90, 121.51, 119.64, 118.70, 118.24, 114.04, 109.08, 76.68, 63.69, 45.08, 37.73, 32.67, 19.91, 19.34, 14.67; IR (KBr), υmax/cm-1: 3044, 2974, 2929, 1667, 1599, 1472, 1309, 1247, 1174, 1045, 838, 742, 610; ESI-MS: m/z (%) = 434 (100) [M+Na] +.
化合物3y:粉色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.51 (d, J = 7.6 Hz, 1H), 7.12-7.37 ( m, 7H), 7.00-7.04 (m, 3H), 6.83 (s, 1H), 4.92 (t, J = 8.0 Hz, 1H), 3.74 (s, 3H), 3.54-3.70 (m, 1H), 2.34 ( s, 3H), 2.29 (s, 3H); 13C NMR (CDCl3, 100 MHz):δ (ppm) : 201.35, 144.80, 141.99, 138.65, 137.30, 134.91, 132.90, 132.26, 130.25, 130.05, 129.77, 128.64, 127.35, 126.66, 126.26, 126.15, 121.93, 119.27, 119.08, 116.67, 109.31, 76.68, 47.58, 37.76, 32.74, 21.36, 21.10; IR (KBr), υmax/cm-1: 3051, 2958, 2917, 1668, 1561, 1466, 1347, 1290, 1199, 1124, 1029, 978, 818, 741, 6890; ESI-MS: m/z (%) = 437 (100) [M+H+], 458 (93) [M+Na] +.
化合物3z:黄色粉末;1H NMR (CDCl3, 400 MHz): δ (ppm) : 7.94 (d, J = 1.6 Hz, 1H), 7.70 (dd, J = 12, 2.0 Hz, 1H), 7.45-7.49 (m, 3H), 7.16-7.26 (m, 5H), 7.00-7.09 (m, 5H), 6.79 (s, 1H), 4.94 (t, J = 8.0 Hz, 1H), 3.66-3.73 (m, 3H), 2.18 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ (ppm) : 196.54, 141.27, 137.40, 137.28, 136.68, 136.56, 134.56, 133.13, 130.59, 130.16, 129.69, 129.06, 127.09, 126.86, 126.13, 124.83, 121.71, 119.47, 118.89, 117.73, 109.20, 45.42, 37.78, 32.70, 19.91, 19.34; IR (KBr), υmax/cm-1: 3085, 3013, 2934, 2891, 1678, 1579, 1463, 1387, 1243, 1197, 1027, 815, 744, 677; ESI-MS: m/z (%) = 458 (100) [M+Na] +.
3. 结果讨论
3.1. 优化反应条件
以吲哚和3-(4-甲基苯基)-1-(4-甲氧基苯基)-1-丙酮在乙腈中的反应为模型,对反应条件进行了优化,实验结果见表1。首先考察了催化剂Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]的用量对反应的影响(Table 1, entries 1-4)。当不加催化剂时,无法得到目标产物(Table 1, entry 1)。当催化剂用量为15 mol%时,催化效率最高,产率可达98% (Table 1, entry 4),考虑到反应的经济性,确定催化剂的用量为10 mol% (Table 1, entry 3)。其次考察了反应时间、溶剂种类等对该反应的影响(Table 1, entries 5-10)。通过筛选,确定反应的最佳条件为:离子液体催化剂[ThiN(CH2)4SO3][p-CH3PhSO3]用量10 mol%,乙腈为反应溶剂,反应时间5 h,反应温度80℃。
3.2. 反应底物普适性研究
在最佳反应条件下,对反应底物的普适性进行研究,结果见表2。研究发现α, β-不饱和酮的苯环上连有供电子的Me、Et、OMe或OEt等基团或是吸电子的卤原子时,反应都能够顺利的进行,所得产物产率为82%~99% (Table 2, 3b-3l, 3o-3x)。当α, β-不饱和酮的苯环上均为双取代时,也能以优秀的产率得到相应的目标产物(Table 2, 3m、3y、3z)。此外,无论是吲哚还是N-甲基吲哚都能与各种不同取代的α, β-不饱和酮平稳的反应。以上结果表明,该反应底物的普适性好。
4. 催化剂的循环使用性研究
在最佳反应条件下,以吲哚和3-(4-甲基苯基)-1-(4-甲氧基苯基)-1-丙酮为模型反应,考察了离子液体

Table 1. Optimization of reaction conditionsa
表1. 反应条件优化a
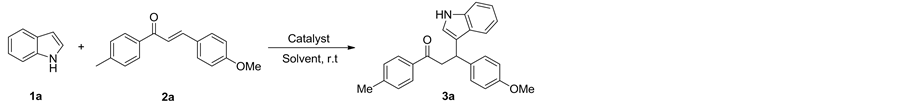
a反应条件:1a (1 mmol),2a (1 mmol),催化剂IL:[ThiN(CH2)4SO3][p-CH3PhSO3],溶剂 (5 mL),80℃;b分离产率。

Table 2. Research of substrate scopea
表2. 底物的普适性研究a
a反应条件:吲哚或取代吲哚(1 mmol),α,β-不饱和酮(1 mmol),[ThiN(CH2)4SO3][p-CH3PhSO3] (10 mol%),80℃,5 h,乙腈(5mL)。b分离产率。
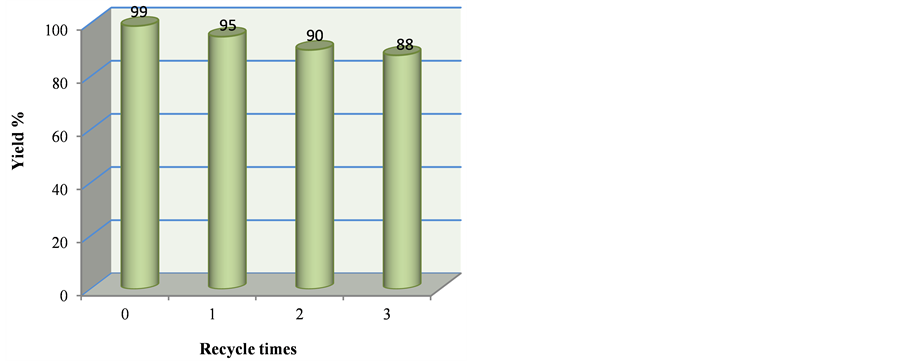
Figure 1. Recycling research of ionic liquid [ThiN(CH2)4SO3][p-CH3PhSO3]
图1. 离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]的循环性研究
催化剂([ThiN(CH2)4SO3][p-CH3PhSO3])的循环使用效果,结果见图1。具体操作是:将15 mL乙酸乙酯分三次加入到除去粗产物后的滤液中萃取残留的有机物,然后将水相中的水旋除,真空干燥至恒重,即得回收的离子液体催化剂,然后直接用于下一次催化循环实验。研究发现该离子液体催化剂循环使用3次,相应产物的产率分别是95%、90%、88%,表明离子液体具有良好的循环使用效果。
5. 总结
本文开展了吲哚和α,β-不饱和酮在Brønsted酸性离子液体[ThiN(CH2)4SO3][p-CH3PhSO3]催化下的Michael加成反应,以82%~99%的产率合成了β-吲哚酮化合物,具有产物收率高、催化剂可循环使用的特点。
基金项目
国家自然科学基金(No. 21572195, 21262035, 21162025)。