1. 引言
帕金森病是中老年人常见的慢性进展性神经系统变性疾病,其主要病理特点是黑质致密部多巴胺(DA)能神经元变性。目前,左旋多巴(levodopa, LD)仍是改善帕金森病症状的金标准药物,但长期应用(一般大于5年)后50%以上的患者会出现异动症 [1] [2] ,主要表现为舞蹈症或手足徐动样不自主运动、肌强直或肌阵挛,可累及头面、四肢和躯干,严重影响了PD患者的生活质量。LID的发生与黑质-纹状体失多巴胺能支配、左旋多巴脉冲样治疗有关,但其确切机制还不完全清楚,目前多认为异动症是由于基底节直接与间接通路失平衡而导致,同时也与多巴胺受体超敏感有关 [3] [4] 。因此明确LD诱发LID的发生机制,寻找LID的防治措施对于改善晚期PD患者的症状,提高生活质量具有极其重要的意义。本研究主要探讨滋肾平颤颗粒对LID大鼠行为学和纹状体多巴胺D1受体活性及基因表达的影响,为进一步深入研究LID的发生机制及防治LID提供理论与实验依据。
2. 实验材料与方法
2.1. 实验药物
滋肾平颤颗粒(熟地黄15 g、枸杞15 g、桑寄生20 g、天麻15 g、僵蚕10 g、莪术15 g、白芍药20 g、天南星15 g、全蝎3 g、蜈蚣3 g)由江苏天江药业有限公司分别制成免煎颗粒剂(批号:1410302),8 g/袋。左旋多巴粉剂(批号:SLD6438)和苄丝肼粉剂(批号:SL03323),购自美国Sigma公司。
2.2. 实验动物
实验动物成年雄性SD大鼠50只,体质量160~180 g,由上海中医药大学动物实验中心提供,动物许可证号为SCXK沪2014-0004。
2.3. 主要试剂和仪器
6-hydroxydopamine (6-OHDA)、apomorphine (APO)、SCH23390、Spiperone,美国Sigma公司产品;3H-SCH23390 (比放射性69·8Ci/mmol)为美国Amersham公司产品;大鼠脑立体定位仪,68001型,深圳瑞沃德;冰冻离心机,Centrifuge 5417R,德国EPPENDORF公司;液体闪烁发光记数仪,Wallacl450型,美国PerkinElmer公司产品。IEppendorf核酸蛋白检测仪,德国Eppendorf。实时荧光定量PCR仪,7500fast,美国ABI;超低温冰箱,MDF-292,SANYO CO。
2.4. PD大鼠模型制备 [5]
SPF级SD大鼠,麻醉后固定于大鼠脑立体定向仪上,参照大鼠脑立体定位图谱 [6] ,采用两点法造模,SNC:前囟后4.8 mm,矢状缝右侧2.0 mm;硬膜下8.0 mm;VTA:前囟后4.8 mm,矢状缝右侧1.2 mm,硬膜下8.2 mm。牙科钻打孔,向两坐标点各注射6-OHDA 6 μg注射速度为1 μl/min,留针10 min。术后14天应用阿朴吗啡腹腔注射(0.5 mg/kg体重),若大鼠恒定转向左侧且旋转圈数 > 7圈/分钟则视为成功帕金森病模型。正常对照组大鼠模型制备:用上述同样方法向右侧SNC和VTA分别注射6 ul生理盐水。
2.5. LID大鼠模型制备
PD大鼠予以左旋多巴腹腔注射治疗四周,注射剂量为20 mg/kg/d,bid。行为学测定AIM评分 > 20分则视为成功的LID模型 [7] 。
2.6. 行为学测试方法
2.6.1. AIM评分测定
AIM评分方法 [8] :AIM评分由4个部分(前肢、口面部、轴性及运动)进行评定,每部分又根据严重程度划分为五个等级(0~4分),其中0分:无;1分:偶尔出现;2分:经常出现;3分:持续存在,刺激使之停止;4分:持续存在,刺激也不能使之停止。
2.6.2. 剂峰旋转次数测定 [9]
各组大鼠在治疗过程中于第7、14、21、28天上午用药后进行大鼠行为学观察和评分。剂峰旋转次数:注射左旋多巴后,每5 min记录旋转次数,最多的旋转次数为剂峰旋转次数。
2.7. 动物分组及给药
42只大鼠造模后经行为学测试,成功造模的PD模型大鼠22只,LD治疗4周后,筛选出成功的LID大鼠模型16只,随机分为模型组、中药干预组每组8只,另取8只生理盐水造模大鼠为正常对照组。异动症模型组继续予以左旋多巴/苄丝肼腹腔注射,每日10 mg/kg,2次/d;中药干预组在模型组处理基础上给予中药灌胃(滋肾平颤颗粒24 g溶于100 ml生理盐水中),每次灌胃量为9 ml/kg,1次/d;正常对照组给予生理盐水灌胃及生理盐水腹腔注射,治疗4周。
2.8. 大鼠脑组织抽提RNA步骤 [10]
取大鼠纹状体组织50 mg,加入1 ml Trizol液,充分匀浆,22℃,静置5 min,加入氯仿0.2 ml,颠倒15 s,22℃,静置3 min,离心:4℃ × 14,000转/分 × 15 min,取上清液0.5 ml,加入0.5 ml异丙醇,混匀,22℃,静置10 min,离心:4℃ × 14,000转/分 × 10 min,弃上液,加入1 ml 4℃ 75%乙醇,离心:4℃ × 10,000转/分 × 5 min,弃上液,真空干燥5 min,用40 ul DEPC处理水溶解,−80℃保存。
2.9. 反转录反应
F(GCTATGCTGACTGGGCTGAC);R(TTTCAGGGATGCTGCCTCT)反转录环境:37℃ 15 min,85℃ 5秒。−20℃保存。PCR反应条件为95℃ 30秒;95℃ 3 s,60℃ 30 s,40个循环。
2.10. RT-PCR数据换算方法
目的基因的相对表达量可以采用∆∆CT法加以分析。CT值—n:扩增循环,∆CT = 目的基因CT − 内参C (注:同一反转录产物扩增后),∆∆CT = 观察样本∆CT − 对照样本∆CT = (观察样本目的基因CT − 观察样本内参CT) − (对照样本目的基因CT − 对照样本内参CT),样本的相对表达量:= 2−∆∆CT。
2.11. 膜蛋白制备
参照张旺明等 [11] 方法制备膜蛋白,最后1次给药2 小时后,大鼠以1%戊巴比妥钠腹腔注射麻醉后,快速断头取脑,于冰块上分离并切取双侧尾壳核脑组织各一块。加入4℃预冷的离心缓冲液,冰浴中制成匀浆,离心后取沉淀以Tris-盐酸缓冲液(pH 7.4) 1~2 ml稀释,充分混悬,即成膜蛋白悬液。以考马斯亮蓝法测定膜蛋白浓度,并调整浓度为1 g/L左右。
2.12. 多巴胺受体活性的测定
采用双复管法(每个样本分为9个不同浓度反应管,重复测定2次)测定,反应管内加入浓度呈倍比递增的标记配基(特异性结合:10、20、40、60、80和100 ul;非特异性结合:20、60和100 ul)和非标记配基(非特异性结合:100 ul)以及定量的膜蛋白液0.2 ml,加入Buffer缓冲液,使每管总反应体积为0.4 m1,37℃水浴中孵育15 min,于冰浴中终止反应。以多头细胞样品收集器收集于玻璃纤维滤纸上,置80℃烘箱中烤干,剪下样品,以2000CA/LL型液态闪烁计数仪测定其放射活性,以空白滤纸点样进行校正。计算各点特异性结合计数,使用受体数据软件包(上海第二医科大学编制),按Scanchard公式计算出多巴胺受体的最大结合容量(Bmax, fmol∙mg)和平衡解离常数(KD, nmol∙L)。
2.13. 统计方法
所有数据以均数士标准差(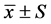 )表示,用SPSS18.0统计学软件分析,(1) 行为学统计:使用重复测量数据多重比较配对的t检验法(Bonferroni法),可进行每个分组每个时间点上作用的两两比较;使用多元方差分析的方法,可进行每个时间点上每个分组之间作用的两两比较。P < 0.05为差异有显著性意义。(2) 多巴胺受体检测:两组间的比较采用独立样本t检验,多组组间差异的显著性采用单因素方差分析进行检验,P < 0.05为差异有显著性意义。
)表示,用SPSS18.0统计学软件分析,(1) 行为学统计:使用重复测量数据多重比较配对的t检验法(Bonferroni法),可进行每个分组每个时间点上作用的两两比较;使用多元方差分析的方法,可进行每个时间点上每个分组之间作用的两两比较。P < 0.05为差异有显著性意义。(2) 多巴胺受体检测:两组间的比较采用独立样本t检验,多组组间差异的显著性采用单因素方差分析进行检验,P < 0.05为差异有显著性意义。
3. 实验结果
3.1. 各组大鼠给药前后神经行为学(AIM评分)的变化
与治疗前(第0天)比较,模型组大鼠用药后第14、、21和28天的AIM评分逐步增高(P < 0.01);中药干预组第14天AIM评分较治疗前升高(P < 0.05),14天后AIM评分逐步降低,至第28天评分显著低于治疗前(第0天)。与同期模型组比较,中药干预组大鼠第7天AIM评分差异无统计学意义,自第14天起的AIM评分值低于同期模型组(P < 0.05)。见表1,图1。
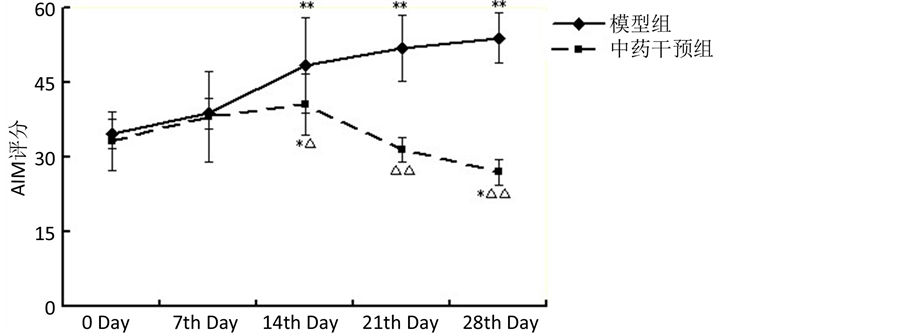
3.2. 各组大鼠给药前后神经行为学(剂峰旋转次数)的变化
与治疗前(第0天)比较,模型组大鼠用药后第7、14、21和28天的剂峰旋转次数逐步增高(P < 0.05),中药组自第14天起剂峰旋转次数逐步增高(P < 0.01),中药干预组大鼠自第14天起的旋转评分值低于同期模型组(P < 0.01)。见表2,图2。

Table 1. Comparison of AIM score between two groups
表1. 两组大鼠AIM评分比较( )
)
 注:与治疗前(第0天)比较,*P < 0.05,**P < 0.01;与模型组比较,△P < 0.05,△△P < 0.01。
注:与治疗前(第0天)比较,*P < 0.05,**P < 0.01;与模型组比较,△P < 0.05,△△P < 0.01。
Figure 1. Comparison of AIM score between two groups
图1. 两组大鼠AIM评分比较
3.3. 各组大鼠双侧尾壳核多巴胺D1受体活性变化
损毁侧模型组、中药干预组与正常对照组比较Bmax显著升高(P < 0.01),模型组上升幅度最大;KD值较之正常对照组,各治疗组均明显降低(P < 0.05);中药干预组与模型组比较,Bmax降低,KD值升高(P < 0.01)。健侧模型组较之正常对照组Bmax升高,KD值降低(P < 0.01),中药干预组较之正常对照组Bmax升高(P < 0.01),其余无明显差异。模型组、中药干预组损毁侧较健侧Bmax升高,KD值降低(P < 0.01)。见表3。
3.4. 各组大鼠双侧尾壳核多巴胺D1受体基因表达变化
损毁侧模型组、中药干预组与正常对照组比较D1受体mRNA表达显著升高(P < 0.05),模型组上升幅度最大;中药干预组与模型组比较,D1受体mRNA表达降低(P < 0.01)。健侧模型组、中药干预组较之正常对照组D1受体mRNA表达明显升高(P < 0.01),其余无明显差异。模型组损毁侧较健侧D1受体mRNA表达升高(P < 0.05)。见表4,图3。

Table 2. Comparison of the rotation times between two groups
表2. 两组大鼠剂峰旋转次数比较( )
)

Table 3. Changes of dopamine D1 receptor activity in the caudate putamen among each group
表3. 各组大鼠双侧尾壳核多巴胺D1受体活性变化( )
)
注:与正常对照组比较,*P < 0.05,**P < 0.01;与模型组比较,△P < 0.05,△△P < 0.01;与健侧比较,#P < 0.05,##P < 0.01。
 注:与治疗前(第0天)比较,*P < 0.05,**P < 0.01;与模型组比较,△P < 0.05,△△P < 0.01。
注:与治疗前(第0天)比较,*P < 0.05,**P < 0.01;与模型组比较,△P < 0.05,△△P < 0.01。
Figure 2. Comparison of the rotation times between two groups
图2. 两组大鼠剂峰旋转次数比较

Table 4. Comparison of relative expression of dopamine D1 receptor mRNA in the caudate putamen among each group
表4. 各组大鼠双侧尾壳核多巴胺D1受体mRNA相对表达量比较( )
)
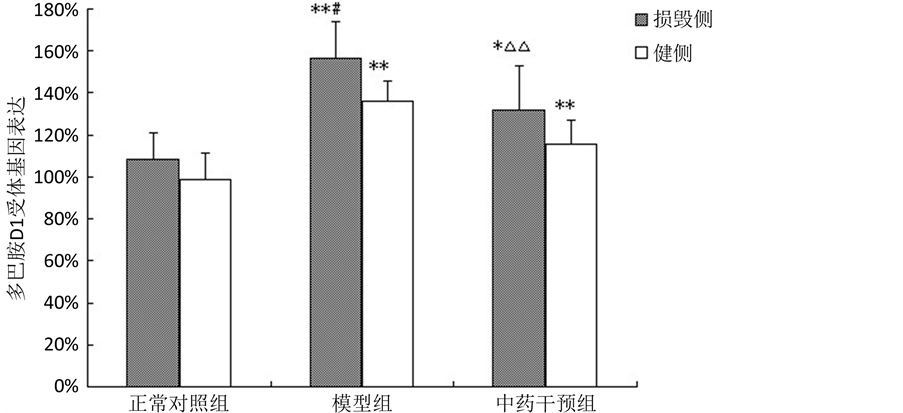 注:与正常对照组比较,*P < 0.05,**P < 0.01;与模型组比较,△P < 0.05,△△P < 0.01;与健侧比较,#P < 0.05,##P < 0.01。
注:与正常对照组比较,*P < 0.05,**P < 0.01;与模型组比较,△P < 0.05,△△P < 0.01;与健侧比较,#P < 0.05,##P < 0.01。
Figure 3. Comparison of the rotation times between two groups
图3. 两组大鼠剂峰旋转次数比较
4. 讨论
目前,关于异动症的发生机制仍不清楚,有学者 [12] [13] 提出LID与纹状体多巴胺Dl和D2受体分别介导的直接通路(兴奋性)和间接通路(抑制性)活动失衡导致的基底节-丘脑-皮质环路活动异常有关。也有学者 [14] 提出LID的发生可能与纹状体内多巴胺D1受体所介导的直接通路功能活化有关。
本次实验结果显示:模型组大鼠随着LD腹腔注射时间的延长,AIM评分进行性升高,剂峰旋转次数亦进行性升高,异动症症状愈发加重。模型组大鼠多巴胺D1受体活性明显增加,基因表达上调,直接通路激活。所以我们认为,LID的发生主要是多巴胺D1受体所介导的直接通路功能活化所引起,当直接通路活化时,由纹状体释放并传递至GPi和SNr的主要神经递质GABA含量增加,对GPi和SNr的抑制作用增强,使后者对丘脑的抑制作用减少,使其释放的兴奋性神经递质Glu增加 [15] ,也进一步使大脑皮层的兴奋性神经递质Glu释放增加,最终导致LID症状的出现 [16] 。研究中还观察到随着LD治疗时间的延长,健侧纹状体亦出现多巴胺D1受体活性及亲和力增加,基因表达上调的现象,说明大鼠DA能神经元可能存在双侧交叉支配现象,6-OHDA破坏一侧DA能通路后,对侧尾壳核交叉支配纤维亦被破坏。
滋肾平颤颗粒源于熟地平颤方,以滋补肝肾、通络解毒为基本思路,是已故上海市名老中医胡建华教授治疗帕金森病的经验总结方。临床应用数十年,治疗PD疗效显著 [17] 。在之前的实验研究中发现:中药可以通过调节突触后多巴胺D1、D2受体表达 [18] ,下调过度激活的ERK凋亡通路 [19] 等发挥神经保护作用。本次试验结果显示,滋肾平颤颗粒通过下调纹状体多巴胺D1受体活性及基因表达,改善基底节区直接通路的过度活化状态,从而有效缓解了异动症症状。
5. 结论
多巴胺D1受体介导的直接通路过度激活可能是帕金森病异动症产生的主要机制。滋肾平颤颗粒主要是通过下调多巴胺D1受体活性及基因表达从而有效缓解异动症症状。
基金项目
国家自然科学基金面上项目:熟地平颤方抑制α-突触核蛋白错误折叠及异常聚集防治帕金森病的深化研究(项目编号:81673726);上海市进一步加快中医药事业发展三年行动计划(项目编号:ZY3-JSFC-2-1007);国家中医临床基地龙医学者-育苗计划:(项目编号:LYTD-34);上海市卫计委杏林新星人才计划项目(ZY3-RCPY-2-2005)。