摘要:
手性是生命体系的基本特征,生命体系具有高度的手性选择性,表面手性可能显著影响生物材料的生物学功能。本文将不同手性酒石酸分子固定在Ti-O膜表面,获得了不同手性表面。XPS检测结果显示,沉积了多巴胺的样品表面出现了新的N1s峰,接枝了己二胺的样品表面N:C值升高,随后又因为接枝了手性酒石酸,其表面的O:C值升高,XPS结果表明各层次表面均已制备成功。水接触角检测结果显示,L-酒石酸表面和D-酒石酸表面的水接触角几乎没有差异,说明表面手性不会影响材料表面的亲疏水性。通过纤维蛋白原变性实验和体外血小板粘附与激活实验来评价手性对于材料表面血液相容性的影响。相对于L-酒石酸表面,D-酒石酸表面显示出较低的纤维蛋白原变性程度,并且血小板的粘附量更少,血小板被激活的程度更低。这些结果表明手性对于生物材料的抗凝血性能有着不容忽视的影响,为开发性能优异的抗凝血材料提供了新的思路。
Abstract:
Chirality is one of the basic characteristics of life system, which has a high degree of chiral selectivity, surface chirality may significantly affect the biological function of biomaterials. In this paper, different chiral tartaric acid molecules were immobilized on the surface of Ti-O films. XPS test results showed the peak of nitrogen appeared on the samples which had deposited dopamine, the ratio of nitrogen to carbon was increased when hexamethylendiamine was introduced. When chiral tartaric acid was grafted, the ratio of oxygen to carbon was increased. XPS results showed that all surfaces had been successfully prepared. The water contact angle test results showed that no difference was present on the L-surface and D-surface, indicating that surface chirality had little effect on surface hydrophobicity. The effects of chirality on the blood compatibility of materials were evaluated by fibrinogen denaturation test and platelet adhesion and activation assay in vitro. Compared with L-tartaric acid surface, D-tartaric acid surface showed a lower degree of denatura-tion of fibrinogen, less platelet adhesion and a lower degree of platelet activation. These results indicate that chirality cannot be ignored in the anticoagulant properties of biomaterials, which provides a new idea for the development of anticoagulant materials with excellent properties.
1. 引言
对于与血液接触材料来说,对于如何提高其血液相容性,研究者的设计目前还是多集中于材料表面的物理和化学性质 [1] [2] [3] ,例如亲疏水性、表面电荷、表面官能团等等,而很少考虑到手性元素。但是,目前已经有一些研究者在研究手性表面对于蛋白质吸附和细胞行为的影响。Rajagopal Appavu将手性氨基酸构成的自组装多肽纳米纤维作为一种免疫佐剂,研究手性对其抗体反应的影响,通过结果可知D-型自组装多肽可以刺激老鼠得到更强的抗体反应 [4] 。为了研究手性表面对于淀粉样蛋白质的影响,Guangyan Qing在类似于细胞膜的氧化石墨烯接枝手性氨基酸,结果表明R型也就是右旋氨基酸抑制β淀粉样蛋白的粘附、成核、纤维伸长过程,而S型则是促进这个过程 [5] 。随着对手性分子在生物体系的作用认识的加深,国内外的研究者也已开展了一些关于表面手性与细胞相容性的相关研究。相对于于L-表面,C-6-神经胶细胞和内皮细胞对D-PEN修饰的3D水凝胶支架显示出了更好的亲和性 [6] 。研究者在金的表面构建了两种不同手性构型的半胱氨酸来研究L929成纤维细胞在其上的粘附行为,结果发现在没有血清加入时,细胞在两个手性表面是没有明显差异的,但是当有血清的存在时,细胞更多的是粘附在L表面上。这种现象表明血清蛋白对于细胞的选择粘附行为可能起着很重要的作用,接着,研究者又用SPR分别研究蛋白质在L和D表面的吸附行为,研究结果表明更多的蛋白质是吸附在L表面 [7] 。胰岛素可以促使神经元PC12细胞增殖分化,因此研究者利用手性酒石酸分子制备手性表面,在预先固定胰岛素的D表面上PC12细胞有高增殖、高分化行为,而在L表面上则没有,推测D表面上的胰岛素具有生物活性,在L表面上则没有,通过其后的AFM检测分析发现胰岛素在D表面保留了其生物活性,而在L表面形成了淀粉样的原纤维,失去了生物活性 [8] 。综上,蛋白质和细胞可能在不同的手性表面具有不同的选择性识别能力。那么,在凝血系统中,表面手性也极有可能存在作用。本文工作就是在Ti-O膜表面通过聚多巴胺和己二胺构成中间层接枝不同手性酒石酸分子,研究表面手性对于材料血液相容性的影响。
2. 实验部分
2.1. 材料和仪器
材料:硅片;新鲜人血(购买自血站);蒸馏水、丙酮、乙二醇试剂均为分析纯;多巴胺、己二胺、L-/D-酒石酸、EDC、NHS、MES、人纤维蛋白原(Sigma-Aldrich);鼠抗人纤维蛋白原γ链单克隆抗体(Abcam);羊抗鼠单克隆抗体/HRP(Bioss)。
仪器:UBMS450型高真空非平衡磁控溅射设备;ESCALB MK-Ⅱ X射线光电子能谱分析(XPS);DSA100接触角测量仪;JSM-7001FJEOL场发射扫描电镜 (FE-SEM);μQuant Biotech酶标仪。
2.2. 表面固定不同手性酒石酸及其表征
利用UBMS450型高真空非平衡磁控溅射设备在Si片上沉积Ti-O膜,将沉积有Ti-O薄膜的硅片切成8 mm × 8 mm尺寸大小,样品标记为Ti-O。配制pH = 8.5,浓度为1.2 mg/ml Tris溶液,随后用Tris溶液溶解多巴胺浓度至2 mg/ml。将多巴胺溶液倒入已先放入样品的培养皿中,将其置于37℃恒温空气浴振荡箱中,反应12 h。通过上述步骤沉积一次聚多巴胺薄膜为一层,重复五次,获得五层聚多巴胺薄膜,样品标记为DOPA,为了在表面引入更多的伯氨基便于后续的反应,利用Tris溶液配制浓度为42 mM己二胺溶液将其倒入样品中,制得富氨基表面,样品标记为HD。用蒸馏水配制浓度为10 mM的酒石酸,在浓度为50 mM的MES缓冲溶液体系下,用EDC/NHS活化羧基,时间5 min,-COOH:EDC:NHS的摩尔比为1:3:5,样品分别标记为HD+L-TA和HD+D-TA。通过XPS实验分析材料的成分。通过躺滴法来测量材料表面的水接触角,评价材料表面的亲疏水性。每个样品测量12个点,取其平均值。
2.3. 纤维蛋白原变性实验
纤维蛋白原是凝血级联反应中至关重要的环节,通过检测纤维蛋白原变性实验可以一定程度反应材料的凝血能力 [9] [10] 。称取适量纤维蛋白原溶于42 g/L的BSA溶液中,使其浓度为3 g/L。吸取500 uL上述混合溶液于样品表面,37℃条件下孵育1 h,PBS清洗、吹干样品。将样品放入1 ml 1%牛血清白蛋白封闭液,在37℃条件下孵育30 min,PBS清洗、吹干样品。将样品移入新培养板,加入20 ul鼠抗人纤维蛋白原γ链单克隆抗体溶液(Ⅰ抗:1% BSA = 1:3000)覆盖样品表面,置于37℃孵化箱孵育1 h,使它与吸附的纤维蛋白原丫链端特异性结合,PBS清洗、吹干样品。将样品移入新孔,加入20 ul标记有辣根过氧化物酶(HRP)的羊抗鼠单克隆抗体溶液完全覆盖材料表面,于37℃培养使之与一抗结合,用PBS洗去未能结合的二抗(Ⅱ抗:1% BSA = 1:5000)。将样品移入新孔,将TMB溶液滴加至样品表面,每样120 ul,进行8 min中的显色反应。显色时间到后,在每个样品滴加50 uL H2SO4终止显色,最后从各样品孔板取100 uL液体置于96孔板,在450 nm波长下利用酶标仪进行读数。
2.4. 体外血小板粘附与激活实验
体外血小板粘附与激活实验是评价材料血液相容性的重要方面。实验结果主要从荧光染色和扫描电镜结果来获得。新鲜人血以1500 rpm 离心15 min后,取上层血浆层-富血小板血浆(PRP)。取500 ul PRP置于样品表面,37℃孵化箱孵育30 min,然后用NaCl溶液清洗样品。用2.5%戊二醛固定样品6 h以上,最后用NaCl溶液清洗样品。取70 ul罗丹明溶液置于样品表面15 min,然后用NaCl溶液清洗样品,吹干样品。将样品置于荧光显微镜400×下观察、拍照和计数。每组平行样荧光照片至少取10张,方便对其进行计数统计。随后将样品置于体积比依次为50%、75%、90%、100%的乙醇/水溶液进行逐级脱水,每次15 min。最后将脱水后的样品喷金,置于扫描电镜下进行形貌观察。本文中涉及的科研用血符合实验规范和伦理要求。
3. 结果和讨论
3.1. 不同手性酒石酸材料的表征分析
图1为Ti-O、DOPA、HD、和HD+L-TA样品的XPS的全谱图,从图中可以看出Ti2p峰在DOPA样品上消失了,新出现了N1s峰,表明在Ti-O样品表面成功地制备了聚多巴胺薄膜,通过宽谱图的O1s、N1s和C1s峰的峰强变化间接地证明各层分子接枝成功。HD和HD+L-TA样品表面所含有的元素与DOPA样品相同,因此在XPS全谱图上可以看到C1s、N1s和O1s。通过表1可以得到各个样品表面的O:C和N:C,同DOPA样品相比较,接枝上HD后,DOPA表面N元素的含量有所提高,这可能是由于己二胺分子比多巴胺含有更多的N元素造成的。接枝上L-酒石酸后,相对于HD样品来说,其表面O:C的值增加,N:C值减少,可能的原因是酒石酸其中一个羧基消耗了HD样品的伯氨基,并且在表面又引入了一个羧基的缘故,因为HD+D-TA与HD+L-TA样品的XPS全谱图相同,因此在这里只展示了HD+L-TA的全谱图。
图2为各样品的水接触角结果,由结果可知没有进行任何处理Ti-O膜表面水接触角为75˚左右,当沉积上聚多巴胺薄膜后,因为引入了大量的亲水性基团如醌基、酚羟基等,使得样品表面亲水性提高。在DOPA样品上接枝己二胺后,HD样品的水接触角变大,亲水性降低,可能是因为己二胺的疏水支链暴露的原因。之后分别在HD样品上接枝不同手性酒石酸后,样品表面的亲水性又再次提高,这可能是因为表面引入了大量的亲水性基团羧基。HD+L-TA和HD+D-TA样品之间水接触角没有差别,说明表面手性并不影响样品表面的亲疏水性。
3.2. 纤维蛋白原变性实验结果分析
如图3所示,纤维蛋白原在HD样品上г链暴露量最大,说明纤维蛋白原变性最严重,这可能是因为HD样品上存在有大量的带有正电的伯氨基基团,而纤维蛋白原又是带负电,使得纤维蛋白原发生了强烈的变性,当L-/D-酒石酸接枝到表面后,因为引入了大量羧基而带有负电,因此样品表面纤维蛋白原变性都有不同程度地减弱。相比于HD+L-TA表面,HD+D-TA表面的纤维蛋白原变性程度要弱。类似地,胰岛素在D-TA表面保持了活性,而在L-TA表面,胰岛素则发生了变性 [8] 。在不同手性表面之间,不同的г链暴露量,这可能是因为不同手性表面构成了不同的空间立体结构,因此导致了不同程度的纤维蛋白原变性。
3.3. 体外血小板粘附与激活结果分析
图4为各样品血小板免疫荧光图,从图中可以看出HD样品表面粘附的血小板量最多,血小板出现了聚集现象,说明其血液相容性不好,这与纤维蛋白原变性的结果也是一致的。图5为各样品血小板计数结果,由结果可知当接枝上L-/D-酒石酸后,血小板粘附数量有所减少,这可能是因为HD+L-/D-TA样品带有大量负电荷的原因。相比于L-酒石酸样品来说,D-酒石酸样品表面的血小板的量明显减少,并且没有出现聚集的现象,说明HD+D-TA样品比HD+L-TA样品有更好的血液相容性,这与纤维蛋白原变性的结果也是一致的。

Figure 2. Water contact angle of samples (n = 6)
图2. 各样品的水接触角

Figure 3. Adsorbed fibrinogen denaturation on samples (n = 4, p* < 0.05)
图3. 各样品纤维蛋白原变性(n = 4,p* < 0.05)

Figure 4. Immunofluorescent images of platelet of samples
图4. 各样品的血小板粘附免疫荧光图
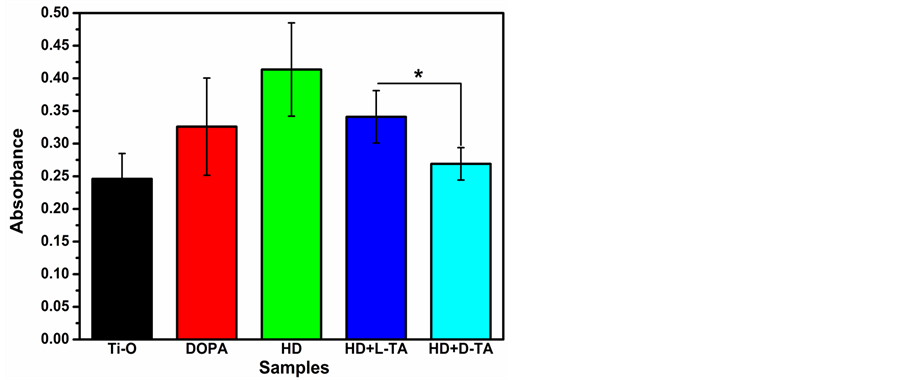
Figure 5. Quantitative analysis of adhered platelets on samples (n = 4, *P < 0.05)
图5. 各样品表面血小板粘附计数结果(n = 4, *P < 0.05)

Table 1. The ratio of oxygen to carbon and the ratio of nitrogen to carbon of samples by XPS spectra
表1. 各样品表面的O:C和N:C
为了进一步观察血小板激活的情况,通过SEM来观察血小板形貌。如图6所示,HD表面血小板呈现出“煎蛋状”,激活现象严重。而接枝了L-/D-酒石酸的样品表面血小板激活程度减弱。HD+L-TA样品表面几乎所有的血小板都伸出伪足,激活情况严重,血小板伸出伪足,而HD+D-TA表面部分血小板伸出伪足,血小板激活程度明显低于HD+L-TA样品表面。

Figure 6. Morphology of platelet adhesion of samples
图6. 不同样品表面血小板粘附形貌(SEM)
4. 结论
通过聚多巴胺和己二胺中间层,将不同手性酒石酸分子固定在Ti-O膜表面,制备了不同手性酒石酸表面,通过XPS的结果表明各层目标分子制备成功。纤维蛋白原变性的实验结果表明,尽管L-酒石酸和D-酒石酸的物理化学性质相同,在材料表面固定量相同,但是HD+D-TA样品表面的纤维蛋白原变性程度要低于HD+L-TA样品。血小板粘附与激活实验结果表明D-酒石酸样品表面的血小板粘附的量少于L-酒石酸样品表面,血小板激活的程度也要低于L-酒石酸样品表面。
致谢
这项工作是由国家自然科学基金(31270020 )、四川省青年科学基金(2013JQ0043)和中央高校基本科研基金(2682016YXZT14)支持。