1. 引言
冠心病是目前全球死亡率最高的疾病,随着生活水平的提供,冠心病的发病率也逐年升高,大大威胁着人类的健康和生命 [1] 。运用微创介入治疗技术在冠脉狭窄部位植入血管支架是目前治疗冠心病的一种有效的手段 [2] 。然而,晚期血栓和再狭窄等并发症的发生影响了血管支架介入治疗的临床远期疗效,因此解决血管支架临床应用中晚期血栓和再狭窄依然是支架开发中的一大难题 [3] 。血管支架植入过程中血管内皮的损伤和炎症导致的平滑肌细胞增生和支架材料的血液相容性不足是再狭窄和晚期血栓发生的一个主要因素 [4] [5] 。因此血液相容性良好,同时能够抑制平滑肌细胞增生的支架材料是开发心血管器械的理想材料。
钛具有良好的机械性能,优异的抗腐蚀性能和良好的生物形容性,被作为医用植入材料进行大量的研究 [6] 。用阳极氧化技术可以在钛金属材料表面制备高度有序的纳米管结构,纳米管的管径为20250 nm,深度可达到1 mm。有研究表明在钛表面的氧化钛纳米管阵列(TNT),能够更有效的促进成骨细胞的粘附和增殖等 [7] ,TNT还可以抑制白细胞的激活和炎症因子的分泌 [8] ,具有较低的免疫原性。Peng等研究发现,TNT还可以选择性的促进内皮细胞的增殖和迁移,同时抑制平滑肌细胞的生长和限制平滑肌细胞的尺寸 [9] 。这些研究表明,这种特殊结构的氧化钛纳米管在新型支架材料或心血管植入器械的开发中具有很大的应用前景。热处理温度对二氧化钛纳米管的晶型结构和表面特性均有一定的影响 [10] ,细胞在不同晶体结构和表面特性材料表面的生长行为差异很大 [11] [12] 。但是目前尚未见关于不同热处理温度对于TNT表面血管细胞生长行为影响的研究。本研究采用3个不同温度对阳极氧化法制得的TNT薄膜进行热处理,研究不同的热处理温度对TNT表面特性、晶体结构和生物相容性的影响。
2. 材料
钛箔,纯度99.5%,厚度50 μm,由陕西宝鸡有色金属有限公司提供;氯化铜、氟化铵,分析纯,由成都科龙试剂有限责任公司提供;所用血液由成都市血液中心提供。
3. 方法
3.1. 氧化钛纳米管薄膜的制备
将钛箔用剪刀剪成4 cm × 5 cm的片材,并依次用丙酮、无水乙醇、去离子水在超声清洗设备中超声清洗各5~10 min,每种试剂清洗三次,除去表面油渍污渍,密封保存备用。
按照如表1所示的浓度配制电解液,以钛箔为阳极,对阴极为石墨,将电压设置为20 V进行阳极氧化处理3 h。
3.2. 热处理
将经过阳极氧化后的样品通过300℃、450℃、600℃的高温处理4个小时,取出样品分别用丙酮、酒精和去离子水超声清洗3次每次3 min,烘干保存备用。
3.3. 薄膜表面形貌,晶体结果和化学组成性能评价
本文采用Perkin Elmer 16PC型X射线光电子能谱仪,对样品表面各种元素的百分含量进行测量;采用Quanta200型环境扫描电子显微镜(荷兰FEI公司),对样品的表面形貌进行表征;本文采用X射线衍射仪(X-Ray Diffraction,XRD,型号:荷兰PhilipsX’ Pert Pro)对材料的晶体结构类型进行分析,采用小角度掠射(0.5),扫描范围20˚~80˚,扫描步长0.03˚。
3.4. 血小板粘附与激活
取新鲜人血并于1500 r/min离心15 min,取上清液,获得富板浆(PRP);各取富板浆50 μL滴加至样品表面,放入恒温水浴过37℃孵育1 h;孵育结束后用PBS清洗样品三次,洗去表面物理吸附的血小板;清洗结束后再用2.5%的戊二醛溶液室温固定过夜;将固定好的样品用PBS洗净吹干,进行P选择素染色观测血小板粘附和激活情况。
LDH法检测血小板粘附定量分析:在各样品表面加入60 μL PRP,并在37℃孵育45 min后用PBS清洗样品表面三次。随后在样品表面滴加40 μL的Triton-X-100 (稀释到1%)处理5 min后,取出25 μL的裂解液加入预先加有200 μL NADH和丙酮酸钠的96孔板中,用酶标仪读取其在340 nm处的吸光度。
3.5. 平滑肌细胞生长行为评价
将取自于人脐动脉的平滑肌细胞分别滴入装有样品的24孔板中,并置于恒温细胞培养箱中培养1 d和3 d。之后,取出样品,吸取细胞悬液,每孔加入350 μL含有10% (体积分数)CCK-8试剂的M199全液。恒温培养箱中孵化4 h后,溶液移入96孔板中,用酶标仪读取其在450 nm处的吸光度。
取出培养平滑肌细胞不同时间的样品,用磷酸盐缓冲液洗去未粘附的细胞,用2.5%的戊二醛溶液固定。在样品表面滴加50 μL罗丹明试剂,避光静置15 min,清洗3次,避光保存。用荧光显微镜观察平滑肌细胞并拍照。
4. 结果与讨论
4.1. 氧化钛纳米管薄膜材料学分析
XPS检测结果如图1所示,经过阳极氧化后钛金属表面出现了C1s、Ti2p、O1s、F1s的光电子峰,其中C和F的引入可能是由于电解液中存在丙三醇和氟化铵,而在阳极氧化的过程中电解液的组分会参与钛纳米管的形成过程,因此出现了C和F的光电子峰。

Table 1. The composition of the electrolyte
表1. 电解液成分
SEM的检测结果显示,钛金属作为阳极在丙三醇和氟化铵的电解液中经过4个小时的氧化过程,在其表面形成了氧化钛的纳米管阵列(图2)。由图a可以发现经过阳极氧化处理获得的纳米管的内径约为80~100 nm,管壁约为20 nm左右。随后对获得的钛纳米管分别进行300℃、450℃和600℃的退火处理。通过退火后的扫描电镜照片可以看出,不同温度的热处理后钛纳米管的结构依然存在,表面形貌、管径和管壁的尺寸变化不大。
XRD结果表明(图3),经过阳极氧化制得的纳米管阵列在退火前没有出现锐钛矿、金红石或板钛矿的衍射峰,证明该方法制得的TNT没有晶体结构是无定型结构,经过300℃热处理后,XRD的结果中出现了锐钛矿晶型的衍射峰,说明经过300℃热处理后,纳米管阵列由无定型结构转变为锐钛矿结构。从450℃热处理后的XRD结果可以看出,450℃退火后氧化钛纳米管中同时出现了锐钛矿和金红石两种晶型结构,其中锐钛矿的晶型结构含量较高,为主要的晶体结构。从600℃热处理的结果可以看出,600℃退火后氧化钛纳米管的结构中同时存在锐钛矿和金红石两种晶型,其中金红石结构含量较高。XRD的结果说明,通过热处理可以使阳极氧化获得的无定型结构的钛纳米管转变为具有一定晶型的钛纳米管阵列。在退火温度较低时,形成的为锐钛矿晶型,而随着退火温度的升高,则逐渐出现金红石型的晶体结构,热处理温度越高金红石的含量越高。这是由于锐钛矿晶型为亚稳相,而金红石晶型为热力学稳定相 [13] 。当退火温度升高时,晶体表面的表面能升高,金红石相在锐钛矿相的界面成核然后逐步扩散至锐钛矿相内部 [14] 。这种转变过程是很缓慢的,因此随着温度升高呈现出金红石相逐渐的趋势。
4.2. 血小板粘附和激活评价
随后,对阳极氧化获得的四种纳米管表面的血小板粘附和激活情况进行了评价,其中图4(a)为各样品的免疫荧光染色,图4(b)为各样品的LDH检测结果。从免疫荧光染色的结果(图4(a))可以看出平板钛金属、未热处理和300℃热处理的钛纳米管表面有大量激活的血小板粘附,并且血小板呈现出大量聚集的状态,证明这三种样品表面的血小板激活程度较高。而450℃和600℃热处理的纳米管表面血小板的激活数量相比与其他三个样品较少,600℃热处理的纳米管表面血小板的激活数量最少。LDH血小板粘附数量检测的结果如图(4(b))所示,看以看出未热处理的TNT表面粘附的血小板的数量明显低于平板钛表
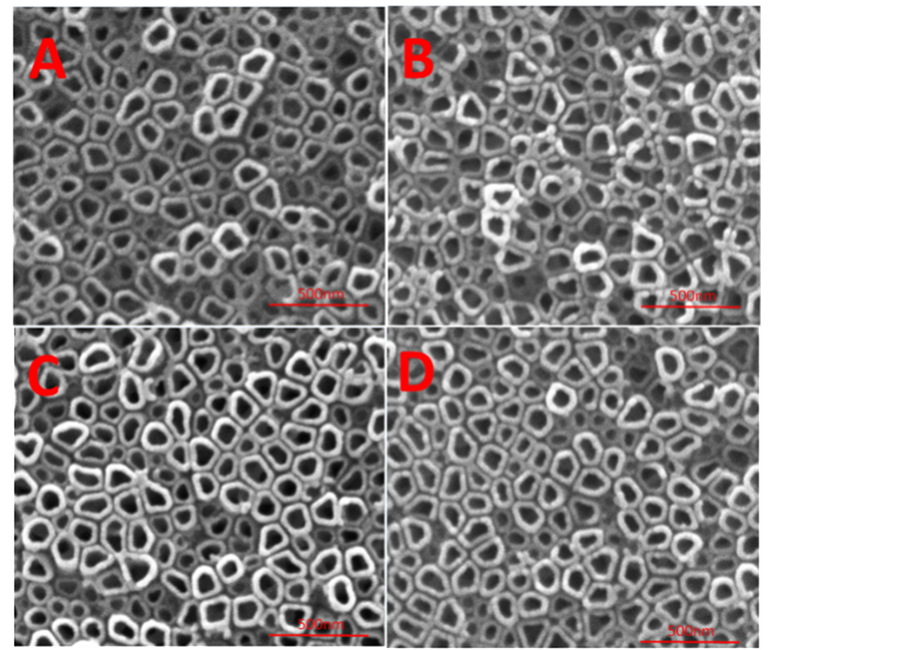
Figure 2. SEM results of TNT and TNT samples by heat treated at 300˚C, 450˚C and 600˚C (A: TNT; B, C, D are TNT samples by heat treated at 300˚C, 450˚C and 600˚C)
图2. TNT与300℃、450℃、600℃不同温度热处理后TNT的SEM结果(A:TNT;B、C、D分别为300℃、450℃、600℃热处理后的TNT)
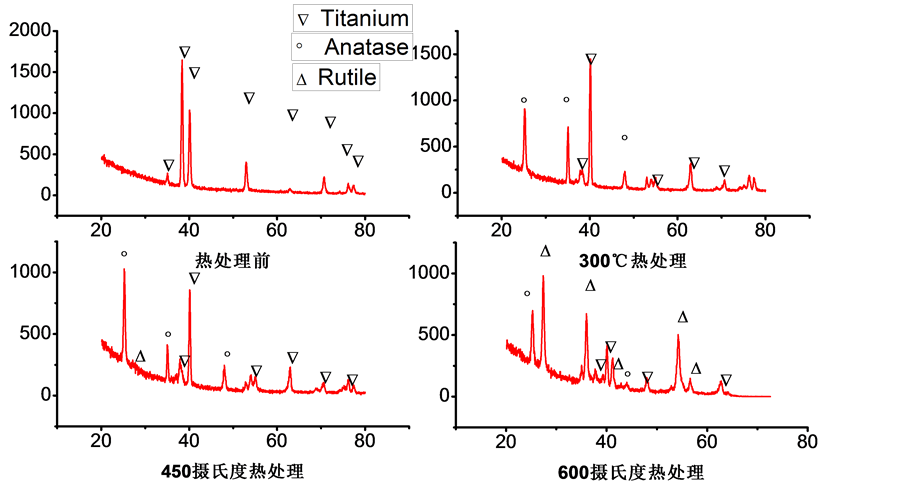
Figure 3. XRD results of TNT and TNT samples by heat treated at 300˚C, 450˚C and 600˚C
图3. 热处理前与300℃、450℃、600℃不同温度热处理后的XRD结果
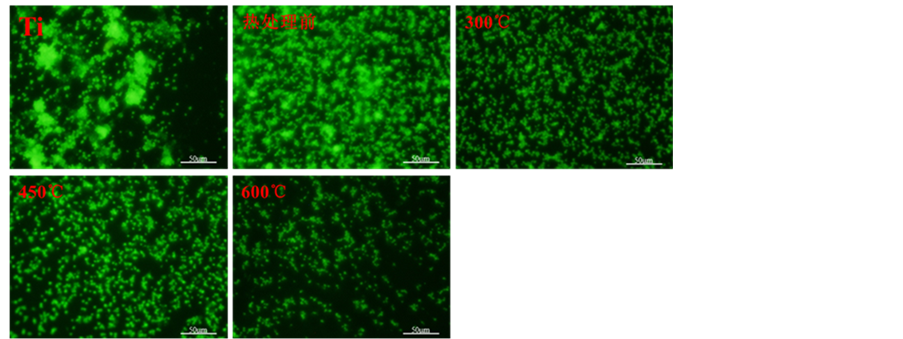 (a)
(a)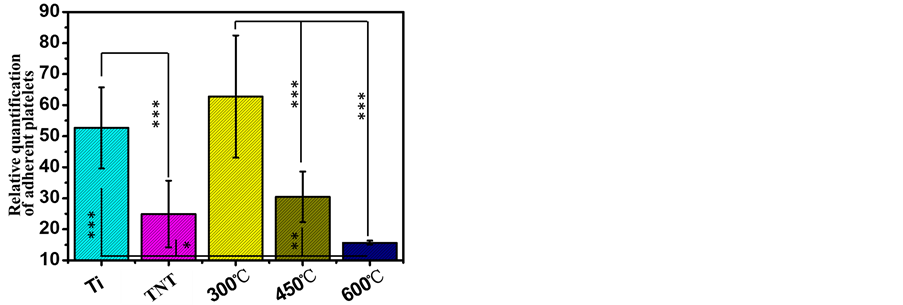 (b)
(b)
Figure 4. Staining of platelets and LDH assay results of TNT and TNT samples by heat treated at 300˚C, 450˚C and 600˚C ((a) Staining of platelets seeded onto TNT and TNT samples by heat treated at 300˚C, 450˚C and 600℃; (b) LDH assay)
图4. 热处理前与300℃、450℃、600℃不同温度热处理后血小板粘附与激活结果。((a) 免疫荧光染色;(b) LDH分析)
面粘附的血小板的数量。而300℃热处理的TNT表面粘附的血小板的数量相比与未热处理的TNT明显增加。而450℃热处理的TNT表面血小板的粘附数量明显少于300℃热处理的TNT表面。600℃热处理的TNT相比与其他4种样品表面血小板的粘附数量最少。血小板免疫荧光染色和LDH检测的结果表明通过不同温度的退火可以调控TNT表面血小板的粘附数量和激活情况,退火温度越高血小板粘附和激活的数量均越少。这可能与不同退火温度得到TNT的晶型不同有关,其中金红石含量高的TNT表面血小板粘附和激活的数量均较少。
4.3. 平滑肌细胞染色和CCK-8结果
样品表面平滑肌细胞罗丹明染色和CCK-8检测结果如图5所示,可以看出培养1天时,相比于平板样品,纳米管表面的SMCs数量明显较少。培养3天时,于1天的荧光照片相比,各样品表面的细胞数量均有所增加,其中平板Ti样品表面的基本被SMCs覆盖,而300℃退火处理的TNT表面的SMCs跟其他样品相比数量较少。培养5天后,平板钛表面的SMCs变得更为细长,同时呈现多层生长的状态。未热处理的TNT表面的SMCs呈现聚集且多层生长的状态,样品表面部分区域无SMCs覆盖。而三种不同温度处理的TNT表面的SMCs显示出不同的生长情况。300℃退火处理的TNT表面的SMCs数量明显少
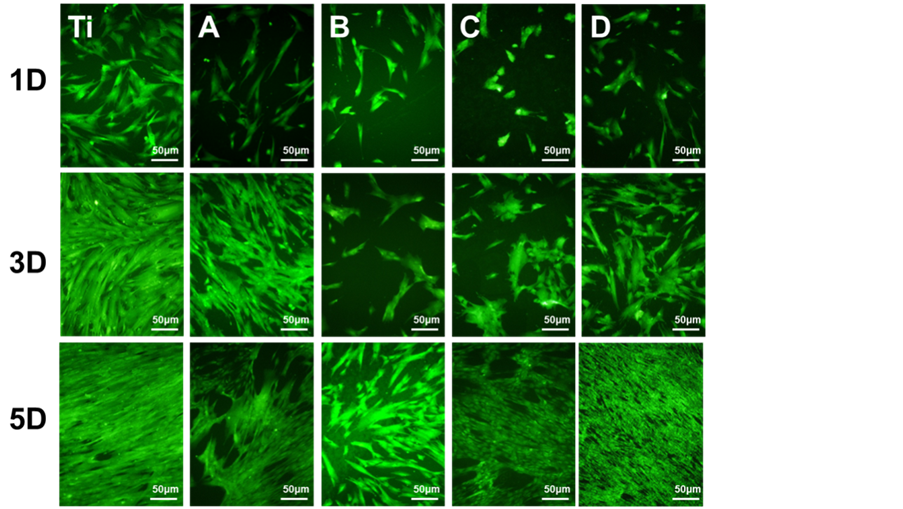
Figure 5. Staining of smooth muscle cells and CCK-8 results of TNT and TNT samples by heat treated at 300˚C, 450˚C and 600˚C (A: TNT; B, C, D are TNT samples by heat treated at 300˚C, 450˚C and 600˚C)
图5. 热处理前与300℃、450℃、600℃不同温度热处理后的平滑肌细胞染色和CCK-8结果(A:TNT;B、C、D组分别为经过300℃、450℃、600℃热处理后的TNT试样)
于另外两组热处理样品。而600℃退火处理的TNT表面基本被SMCs所覆盖。从CCK-8的结果可以看出,培养1天时,与平板钛相比,TNT样品表面的SMCs增殖活性均低于平板钛表面。而三种不同温度退火处理的TNT表面SMCs的增殖活性以此为600℃ > 450℃ > 300℃。不同时间点的样品表面的SMCs生长情况与1天的生长规律一致。SMCs培养的结果说明TNT的热处理温度能够影响其表面SMCs的生长行为,这可能与不同温度退火处理得到的TNT薄膜的晶型不同相关。研究表明,不同晶型的氧化钛其表面血浆蛋白的粘附行为不同,没有经过热处理的氧化钛表面在12 h后有比热处理过后的氧化钛表面更少的蛋白吸附量 [15] 。因此这三种退火温度得到的TNT可能通过调控血浆蛋白在其表面的粘附行为来干预SMCs的生长。
血栓及再狭窄是目前心血管材料临床应用中的两大难题,影响了心血管器械的远期疗效。材料表面血液相容性不足是心血管材料表面血栓产生的一个主要因素 [16] 。血小板与心血管材料相互作用是研究材料血液相容性的一个重要方面。因此血小板的粘附和激活行为是评价材料血液相容性的一个重要指标 [17] 。而再狭窄的产生则是由于血管中膜层的SMCs的过度增生和向内膜层的迁移 [18] [19] 。上述血小板粘附和激活结果和SMCs增殖结果表明,高温600℃热处理得到的金红石晶型的TNT表面粘附和激活的血小板数量更低,可能具有减少由于血小板活化而引起的凝血行为,具有更好的血液相容性。而300℃退火处理得到的TNT表面SMCs具有较低的增殖能力,能够减少SMCs的过度增殖,减少血管内膜的增生,对于减少内膜增生而引起的血管再狭窄具有积极的作用。以上研究结果表明锐钛矿相的TNT具有更好的血液相容性,而金红石相的TNT能够抑制SMCs的增殖有利于抑制再狭窄。
5. 结论
本研究对采用阳极氧化方法制备的TNT进行300℃、450℃和600℃三个不同稳定的热处理,得到了三种不同晶型的TNT薄膜,300℃退火得到的锐钛矿型的TNT表面具有良好的血液相容性,而600℃退火得到的金红石晶型为主的TNT能够抑制SMCs的增殖对于抑制再狭窄具有积极的作用。本研究通过改变TNT薄膜的晶型来调控其表明的血液相容性和细胞生长行为,可以为TNT的在心血管器械中应用提供支持。