1. 引言
脑胶质瘤约占颅内肿瘤的45%,具有生长速度快、高侵袭性等特点 [1] ,并且由于血脑屏障(BBB)的存在,用手术、放化疗等传统治疗手段很难治愈 [2] 。脂质体具有无毒性、低免疫原性等特性,用亲水性的聚乙二醇长链修饰脂质体,可显著提高其体内循环时间 [3] 。在脑靶向药物递送系统中,低密度脂蛋白受体相关蛋白1 (LRP1)常被用于促进受体介导的转胞吞作用 [4] ,LRP1在脑毛细血管内皮细胞和脑胶质瘤细胞上高表达 [5] [6] 。Angiopep-2 (Ang)肽对LRP1具有高度亲和性、TAT肽是一种公认的高效细胞穿膜肽 [7] ,Angiopep-2和TAT双配体修饰的脂质体(Ang-TAT-LIP),可高效地穿透BBB并进一步靶向脑胶质瘤细胞 [8] 。
本课题通过正交试验研究脂质体的优化制备条件,并用透射电镜(TEM)和纳米粒度仪测试其颗粒形貌、平均粒径和Zeta电位等参数。用核磁共振氢谱(1H-NMR)研究Ang/TAT与脂质体的偶联情况。最后用高表达LRP1的人脑星形胶质母细胞U87和大鼠脑毛细血管内皮细胞bEnd.3 [9] ,进行香豆素6荧光脂质体的细胞摄取实验,以表征脂质体对BBB和脑胶质瘤细胞的体外靶向入胞效果。
2. 材料与方法
2.1. 试剂与仪器
Ang和TAT由吉尔生化有限公司合成;大豆磷脂(SPC)购自Lipoid GmbH;胆固醇(CHO)购自Avanti Polar Lipids;PEG修饰的和马来酰亚胺接枝的二硬脂酰磷脂酰乙醇胺(DSPE-PEG2000和DSPE-PEG2000-Ma)买于Nanocs;香豆素6购自于Sigma-Aldrich;Dulbecco改良的Eagle培养基(DMEM)和胎牛血清(FBS)买于Gibco;其它相关的化学药品都为分析纯。
旋转蒸发仪:SY-2000,中国上海亚荣;PVDF针头式过滤器:0.22 μm/0.10μm,Millipore;马尔文纳米粒度仪:Nano ZS90,马尔文仪器有限公司;超声波细胞破碎仪:scientz-IID,新芝生物科技有限公司;TEM:JEM-100CX,日本电子株式会社;核磁共振波谱仪:600 MHz,Unity Inova光谱仪;荧光倒置显微镜(IFM):IX-71,Olympus公司。
2.2. DSPE-PEG2000-Ang/TAT的合成
如图1所示,通过Ang中半胱氨酸的巯基和DSPE-PEG2000-Mal的马来酰亚胺(Mal)之间的共价反应合成DSPE-PEG2000-Ang。简而言之,把DSPE-PEG2000-Mal、Cys-Ang和催化剂三乙胺(摩尔比= 1:1:3)共混于三氯甲烷中,室温避光反应20 h,用氮气防止氧化,用旋转蒸发仪真空干燥混合物。以CDCl3作为溶剂,通过1H-NMR鉴定最终产物。用TAT-Cys替代Cys-Ang,并使用同样的方法合成DSPE-PEG2000-TAT。
2.3. 脂质体的制备
用薄膜分散法制备脂质体 [10] ,如表1所示,将脂质体原材料溶解到三氯甲烷中,旋转蒸发并真空干燥2 h,彻底除去有机溶剂后得到多室脂质体薄膜。将薄膜在50˚C的条件下水化30 min,随后用100 W的功率探头超声(5 s超声,5 s间隔),最后将脂质体溶液挤压通过PVDF滤器。
制备聚乙二醇脂质体(PEG-LIP)是制备其它功能化脂质体的基础,因此用L9(34)正交试验来探究脂质体的优化制备条件,其中旋转蒸发的温度、转速,超声时间和PVDF滤器的孔径被选为影响因素。
基于上述的操作和优化制备条件,制备了四种香豆素6(C6)包载的荧光脂质体(C6:磷脂= 1:80, w/w),包括C6包载的脂质体(C6-LIP)、单配体修饰的C6脂质体(C6-TAT-LIP、C6-Ang-LIP)和双配体修饰的C6脂质体(C6-Ang-TAT-LIP)。
2.4. 脂质体粒径和形貌的表征
分别将脂质体稀释到质量分数为1%和0.1%,用马尔文纳米粒度仪测量脂质体的平均粒径和Zeta电位。将脂质体用超纯水稀释到约1.6 mg/mL,磷钨酸负染脂质体后,用TEM表征脂质体的形貌。
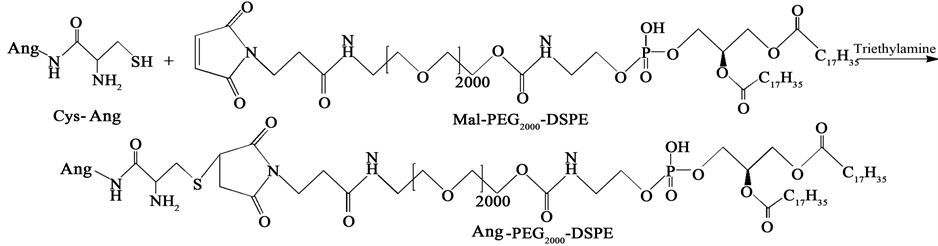
Figure 1. Synthesis of DSPE-PEG2000-Ang
图1. DSPE-PEG2000-Ang的合成

Table 1. Composition of Liposomes (mol%)
表1. 各种脂质体的组成
2.5. 脂质体稳定性的表征
为了研究脂质体的稳定性,可以在FBS中检测脂质体的平均粒径和透光率的变化 [11] 。简而言之,把50 μL各种配方的脂质体加入到1 mL的含有10% FBS的培养基中,然后在37˚C、30 rpm的振荡条件下,置于水浴摇床中温育0~24 h。通过脂质体在不同时间的透光率和平均粒径的变化,反映脂质体的稳定性高低。用酶标仪在750 nm的波长下测试脂质体的透光率变化,用纳米粒度仪表征粒径的变化。
2.6. 体外细胞摄取实验
用DMEM培养基,在37˚C、5% CO2的条件下培养U87细胞和bEnd.3细胞。以2.5 × 104细胞/孔的密度,把细胞接种在24孔板中。培养24 h之后,将不同配方的C6脂质体与细胞共培养4 h,其中C6的浓度为3 μg/mL。再用PBS溶液将细胞清洗三遍,4%的多聚甲醛固定20 min后用荧光倒置显微镜对细胞成像。所有培养基中含有10% FBS、100 U/mL青霉素和0.1 mg/mL链霉素。
2.7. 统计分析
所有的数据都来自三个独立样本,表示成平均值±标准差的形式。用SPSS 22软件单因素方差分析对结果进行分析,p值<0.05和<0.01分别被认为是有统计学差异和有显著性差异。
3. 结果与讨论
3.1. DSPE-PEG2000-Ang/TAT的合成
通过巯基和马来酰亚胺之间的共价连合成DSPE-PEG2000-Ang/TAT。图2(a)为DSPE-PEG2000-TAT的1H-NMR图(溶剂为CDCl3)。4.67 ppm处的特征吸收峰为溶剂残留峰,3.59 ppm处的峰值为PEG的吸收峰,表明亲水性PEG长链的存在,Mal基团的特征吸收峰位于6.72 ppm处。从DSPE-PEG2000-TAT的谱图中可以发现Mal特征吸收峰的消失,这说明DSPE-PEG2000-Mal的Mal官能团和TAT的巯基发生了化学反应,因此在2.2~3.65 ppm处可以发现反应后的重叠峰。核磁共振氢谱表明DSPE-PEG2000-TAT被成功的合成了。同理如图2(b)所示,也可以说明DSPE-PEG2000-Ang的成功合成。
3.2. PEG-LIP的优化制备条件
第一组实验的平均粒径最高,为177.63 nm,同时第五组实验的平均粒径最低,为113.86 nm。极差
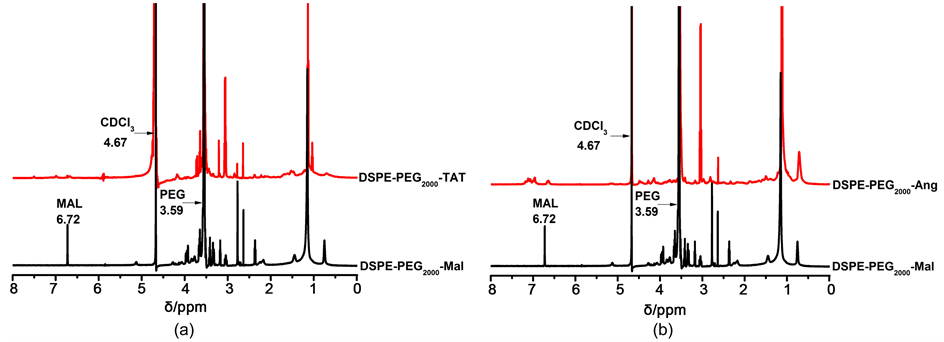
Figure 2. 1H-NMR spectra (inCDCl3) of DSPE-PEG2000-Mal and DSPE-PEG2000-Ang/TAT
图2. DSPE-PEG2000-Mal和DSPE-PEG2000-Ang/TAT的1H-NMR图(溶剂为CDCl3)
越大说明影响因素对系统表现的影响越大 [12] ,由表2可知温度、转速、超声时间和过滤的极差依次为:5.22、5.06、53.49和9.2,因此四个因素对平均粒径的影响高低为:超声时间 > 过滤 > 温度 > 旋转速度。说明探头超声可以有效地分散团聚的小单室脂质体;同时过滤可以除去少量粒径较大的大单室脂质体,减少平均粒径。脂质体在旋蒸时转速的高低,会改变脂质体颗粒的分散程度,影响颗粒周围接触到原子和悬空键的数量、改变脂质体自组装过程中的表面能,从而影响脂质体的粒径,但实验结果说明其影响作用最不显著。
三个水平之间的比较(K1, K2, K3)表明脂质体的优化制备条件为:旋蒸水浴温度为50˚C、旋蒸转速度为120 rpm、探头超声时间为16 min、并且依次过0.22 μm和0.10 μm的滤膜。L9(34)正交试验符合最优化平均粒径的需求,优化制备条件将用于其它脂质体的制备。
3.3. 脂质体的粒径分布和形貌
根据上述优化制备条件,得到PEG-LIP的平均粒径约为99.83 nm,四种C6包载的荧光脂质体的平均粒径为103.66~110.72 nm。脂质体的多分散系数(PDI)均在0.2以内,表明脂质体的粒径分布较均匀、分散性好。PEG-LIP的Zeta电位最小,为−8.65 mV,当其表面偶联了带正电荷的TAT肽(等电点12.13)和Ang肽((等电点9.31)后,Zeta电位值增加,因此C6-Ang-TAT-LIP的Zeta电位最大,为−4.80 mV。
图3(a)和图3(b)为C6-Ang-TAT-LIP脂质体的粒径和Zeta电位的分布,可见其粒径分布较集中,Zeta电位接近于0,表面略带负电。图3(c)为C6-Ang-TAT-LIP脂质体的TEM图,脂质体大小较均匀、为球形的小单室脂质体、没有粘连现象。说明本研究所采用的脂质体制备条件和方法可行。
3.4. 脂质体的血清稳定性
脂质体的表面通常会用PEG修饰以提高稳定性,FBS经常被用于进行生理条件的模拟 [13] 。如图4(a)

Table 2. Orthogonal test results of PEG-LIP preparation
表2. PEG-LIP制备正交实验的结果
和图4(b)所示,24 h内四种脂质体在FBS溶液中透光率和粒径都没有显著的变化,说明脂质体的稳定性良好,在血流中不会有明显的集聚。
3.5. 体外细胞摄取实验
图5为U87细胞和bEnd.3细胞与C6脂质体细胞共培养4 h后的细胞摄取情况,用荧光倒置显微镜进行观察结果,绿色为脂质体中C6的荧光。可见C6-Ang-TAT-LIP的荧光强度比C6-LIP和单配体脂质体(C6-Ang-LIP或C6-TAT-LIP)都要高很多,表明双配体修饰脂质体可以更高效地与U251细胞及bEnd.3细胞结合并被细胞摄取。但通过对比图5(a)和图5(b),可以发现每组脂质体中,U251细胞的荧光强度高于bEnd.3细胞,说明U251细胞对各种脂质体的摄入量大于bEnd.3细胞的摄取量。
实际上,由于LRP1受体在U251和bEnd.3中都高表达,在Ang与受体LRP1特异性靶向结合后,保持了C6-Ang-TAT-LIP与U251及bEnd.3细胞的紧密接触,有利于发挥TAT肽的穿膜作用,进而增强细胞的摄取 [14] ,这也表明Ang与TAT双配体修饰的脂质体具有亲合–穿膜的协同效应。
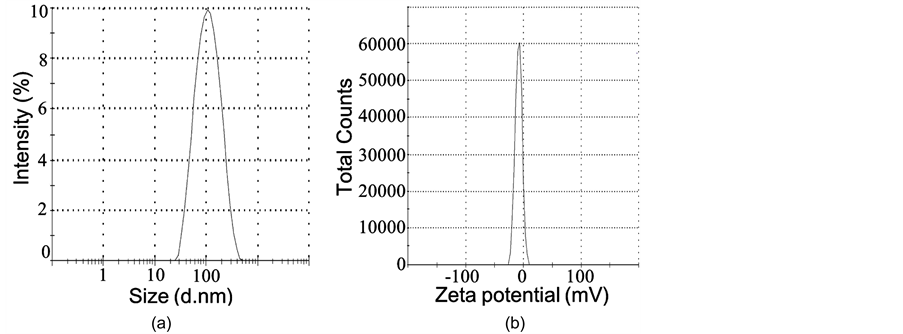
Figure 3. Size (a) and Zeta potential (b) distribution, and TEM graph of C6- Ang-TAT-LIP
图3. C6-Ang-TAT-LIP脂质体的粒径(a)、Zeta电位(b)分布和TEM图(c)
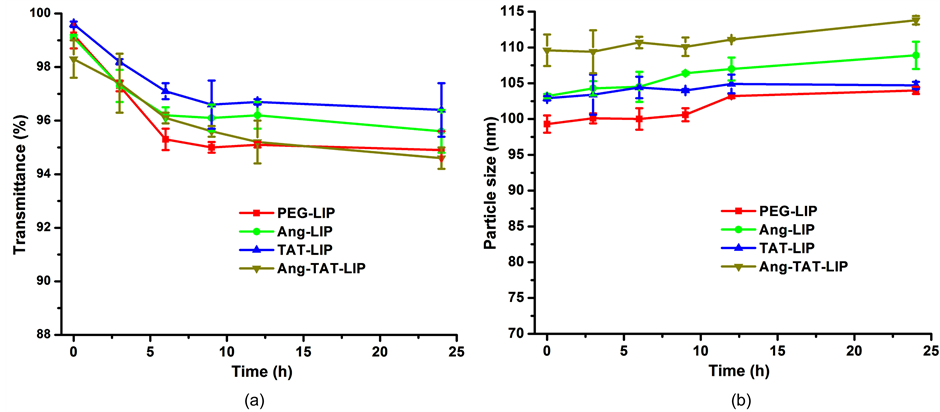
Figure 4. Variations in particle size (a) and transmittance (b) of liposomes in 10% FBS (n = 3)
图4. 脂质体在10% FBS中粒径(a)和透光率(b)的变化(n = 3)

Figure 5. Fluorescent images of C6-loaded liposomes on cells, U87 cells (a), bEnd.3 cells (b). I: C6-LIP, II: C6-Ang-LIP, III: C6-TAT-LIP, IV: C6-Ang-TAT-LIP
图5. C6脂质体的细胞荧光图像,U87细胞(a)、bEnd.3细胞(b);I:C6-LIP,II:C6-Ang-LIP,III:C6-TAT-LIP,IV:C6-Ang-TAT-LIP
4. 结论
本课题通过正交试验研究了脂质体的优化制备条件,用旋转蒸发法制备了双配体修饰的脂质体(Ang-TAT-LIP),并通过1H-NMR证实多肽的成功偶联。脂质体平均粒径约为110 nm,颗粒大小均匀、分散性和血清稳定性较好。体外细胞摄取实验表明Ang-TAT-LIP可以在体外特异性靶向LRP1高表达的脑胶质瘤细胞,并高效地进入细胞内部,是一种具有应用潜力的脑胶质瘤靶向脂质体药物载体。
基金项目
国家自然科学基金项目(No. 51372157)。
NOTES
*通讯作者。