1. 引言
纳米材料具有独特尺寸效应、表面效应、量子效应等纳米效应,具有许多独特的物理化学性质 [1] [2] [3] [4] 。在生物、材料、能源、环境等行业有着广泛的应用 [5] [6] [7] 。如何能够高精度可控重复合成满足特定需求的纳米材料和纳米结构,至今仍然被认为是一个艰巨的挑战。纳米材料与常规材料相比有着其独特的生长特点:纳米材料是在非平衡态下受到热力学和动力学共同作用下生长的;体系中任何局部发生微小的变化均会导致随机成核与随机生长;在常规环境下其生长取向和生长极性存在一定的可能性,但体系内任何因素的改变均会导致产生巨大的差异;生长方向的可变性导致了纳米材料形成一维或者多维结构的材料,其生长过程不同会导致形貌和尺寸的差异;生长参数不同,生长动态过程也必然不同,但最终的形貌必然符合热力学原理。因此研究纳米材料原位生长的原位信息,对于指导纳米材料的可控合成和纳米材料生长机理的研究具有重要的科学意义。
迄今,采用原位技术研究纳米材料生长的主要有:① 原位电镜技术 [8] 、② 扫描隧道显微镜实时观测技术 [9] 、③ 椭圆偏振技术 [10] 、④ 原位X射线表征技术 [11] 、⑤ 原位光谱技术 [12] 、⑥ 原位石英晶体微天平技术 [13] 、⑦ 原位透射电镜技术 [14] 、⑧ 原子隧道显微镜技术 [15] 。以上技术方法均无法获取热力学生长参数描述纳米材料生长过程的瞬时变化动态精细信息;如使用XRD技术研究纳米粒子的生长动力学,仅对球形粒子适用,原位电镜技术、扫描隧道显微镜技术检测条件苛刻缺乏普适性等。现代微量热技术能够高精度、高灵敏度快速准确获取原位过程的热力学信息和动力学信息的独特优势;根据获取的原位热谱信息结合成熟的热动力学理论,可用于推测纳米材料生长过程的化学反应、成核、生长等过程机理。因此采用原位微量热技术,研究纳米材料原位生长机理是具有重要的科学意义。
本文采用高精度、高灵敏度的RD496-III型微量热计获取了双微乳液体系内不同的ω、c、反应物离子比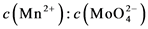 等参量下纳米MnMoO4原位生长过程的热曲信息,结合热动力学理论讨论了不同条件下纳米MnMoO4的生长热动力学参数的变化规律,讨论了纳米材料生长过程的不同阶段的热动力学机理,纳米材料的可控制备及原位生长规律提供实验依据和理论支持。
等参量下纳米MnMoO4原位生长过程的热曲信息,结合热动力学理论讨论了不同条件下纳米MnMoO4的生长热动力学参数的变化规律,讨论了纳米材料生长过程的不同阶段的热动力学机理,纳米材料的可控制备及原位生长规律提供实验依据和理论支持。
2. 实验部分
2.1. 实验试剂与仪器
Na2MoO4·2H2O、Mn(Ac)2、TritonX-100、正辛醇、环己烷、无水乙醇、丙酮(均为分析级)、RD496-III型微量热计(四川绵阳中物热分析仪器有限公司)。
2.2. 原位生长实验
实验步骤:1) 准确配制两份正辛醇比TritonX-100摩尔比为2.57溶液分别向其中加入一定量的Mn(Ac)2和Na2MoO4溶液记为A和B,以600 rad/min搅拌30 min;2) 准确移取1 ml A和1 ml B溶液于微热量仪内外套管中,设置温度待基线稳定后捅破内管反应开始并记录数据;3) 反应完成后分别用丙酮、去离子水、无水乙醇洗涤数次,将产物于真空干燥箱60℃干燥6 h。
根据实验的影响因素设计了不同的实验参数,分别为水/表面活性剂比ω、反应物浓度c、以及反应物离子浓度比RM,具体见表1。
3. 实验结果与讨论
3.1. ω对纳米MnMoO4的形貌尺寸及热动力学参数的影响
ω实验参数的改变对纳米MnMoO4的形貌尺寸的影响结果如表2所示。
图1(a)为1~3样品原位生长的热谱曲线,由图可知纳米MnMoO4在不同生长参数下其热功率变化具有相同的趋势,均经历了放热–吸热–放热三个阶段。其热变机理;双微乳液体系中两种微乳液相互混合,由于布朗运动胶束间会发生相互碰撞、融合、重组、分离等过程,当含有的Mn2+和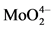 的胶束相融合时会立即发生反应形成产物分子同时释放出大量的热量;一旦体系内分子浓度达到饱和时会形成大量大小不一的晶核;晶核的溶解和生长符合Ostwald熟化理论 [16] ,即小粒子会溶解吸热,大粒子继续生长放热,为吸热和放热竞争的过程热变曲线表现为快速吸热、慢速吸热最后放热由缓慢到平稳的一个过程。依据不同阶段的热谱曲线结合热动力学理论可获取不同阶段的反应速率、反应吉布斯自由能等热动力学参数具体如表3所示。
的胶束相融合时会立即发生反应形成产物分子同时释放出大量的热量;一旦体系内分子浓度达到饱和时会形成大量大小不一的晶核;晶核的溶解和生长符合Ostwald熟化理论 [16] ,即小粒子会溶解吸热,大粒子继续生长放热,为吸热和放热竞争的过程热变曲线表现为快速吸热、慢速吸热最后放热由缓慢到平稳的一个过程。依据不同阶段的热谱曲线结合热动力学理论可获取不同阶段的反应速率、反应吉布斯自由能等热动力学参数具体如表3所示。
由表3可知,样品1和3的第一阶段放热速率k1均大于其第二阶段的放热速率k2,k1越大越利于体系内晶核的形成(具体放热速率见表3)。样品2对应的k1、k2远小于样品1和3,但从热谱曲线可发现其两个放热阶段的放热峰值均明显大于样品1和3。由此推测该条件下ω = 10可能是一个控制形貌尺寸演

Table 1. Determination of microemulsion parameters
表1. 微乳液法参数的设定

Table 2. Reaction conditions and products
表2. 反应条件及产物

Table 3. Thermodynamic parameters of in situ growth process of MnMoO4 nanostructures
表3. MnMoO4纳米材料原位生长过程的热动力学参数
Figure 1. (a), (b), (c), (d), (e), (f) are the FE-SEM patterns of sample 1, 2, 3, 4, 5, 6, respectively
图1. (a)~(c)为样品1~3,(d)~(f)为样品4~6的SEM图
变的临界值,小于10易形成链条状产物且粒径较小;大于10易生成棒状产物且粒径较大。
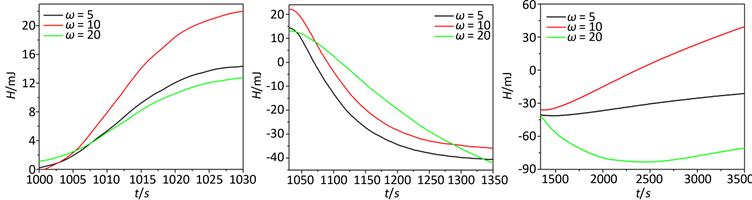
依据原位生长过程的热谱信息,计算出热焓随时间变化规律,依据焓变信息可将纳米材料的原位生长过程分为三个阶段分别为:(a) 反应成核阶段(1000~1030 s),(b) 结晶阶段(1031~1350 s),(c) 晶体生长阶段(1351~3500 s),具体如图3所示。
图1(d)~(f)为样品4~6的SEM图,从表2可知ω由5~20,纳米棒的直径随着ω的增大而减小。图2(b)为样品4~6对应生长过程的原位热谱曲线,发现其曲线趋势与样品1~3具有一致性。但与样品1~3反应条件相比体系内反应物浓度增大,当增加ω值增大了水核半径,使水核间的物质交换速率加快,对应的放热速率k1增大,表3中样品1与4的第一阶段放热k1相比也可以进行说明。样品5的形貌有棒状和块状,其特殊性说明纳米材料非平衡生长的随机性和复杂性有待结合其它技术手段进一步研究。样品6在5420~6010 s之间出现了较明显的放热现象,可能是纳米材料在生长过程的自组装效应而导致。
3.2. 反应物浓度对产物形貌尺寸及热动力学行为的影响
实验参数c(Mn2+)的改变对纳米MnMoO4的形貌尺寸的影响结果表4所示。
图4(a)~(d)为样品1~4的SEM图,c为0.01~0.05 mol/L–1,产物尺寸随c的增大而减小,但当c为0.1 mol/L–1时,可能由于体系浓度过高,导致成核大小不一致,产物发生团聚使尺寸增大。
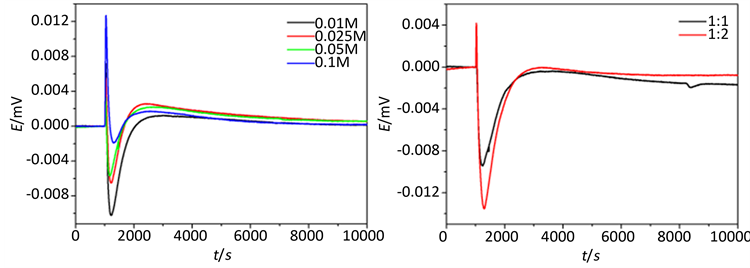
图5中(a)为1~4号样品的生长过程的原位热谱曲线与前面其它条件下的热变趋势相同,过程的热动
 (a) (b)
(a) (b)
Figure 2. (a) is the E-t curve of sample 1, 2, 3; (b) is the E-t curve of sample 4, 5, 6, respectively
图2. (a) 样品1~3的E-t热图谱;(b) 样品4~6的E-t热图谱
 (a) (b) (c)
(a) (b) (c)
Figure 3. The E-t curve of sample 1, 2, 3 different stages, respectively
图3. 样品1~3为不同阶段的热焓时间H-t图

Table 4. Growth parameters of MnMoO4 nanometer materials in double microemulsion system
表4. 双微乳液体系MnMoO4纳米材料的生长参数
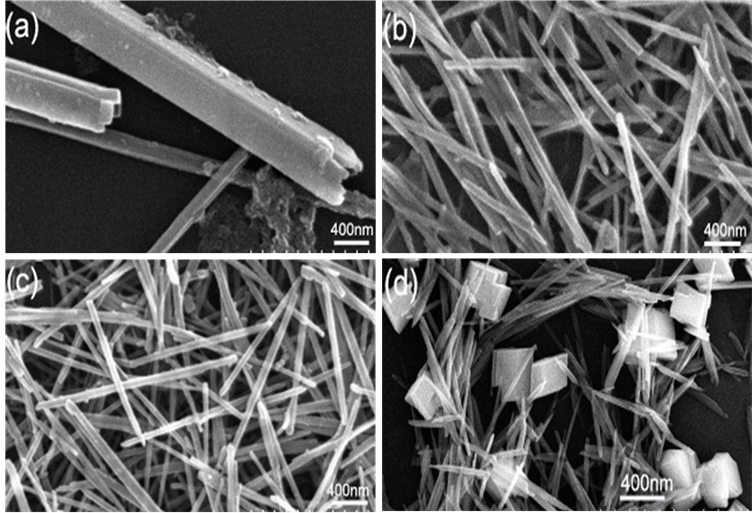
Figure 4. (a), (b), (c), (d) are the SEM patterns of sample 1, 2, 3, 4, respectively
图4. (a)~(d)分别为1~4号样品的SEM图
 (a) (b)
(a) (b)
Figure 5. (a) is the E-t curve of sample 1, 2, 3; (b) is the E-t curve of sample 4, 5, 6, respectively
图5. (a)为样品1~4的E-t 图谱;(b)为样品1和样品2的E-t图谱
力学参数如表5所示。1~3号样中,第一阶段放热峰值与放热速率k1随着Mn2+的浓度c(Mn2+)的增大而减小,这是由于反应物浓度增加,反应粒子数目增多,有效碰撞几率增大,化学反应速率加快所致。随后的吸热阶段的吸热峰值随着浓度增大成递减趋势,是由于产物分子成核后Ostwald熟化的进行产生的吸热效应和第一阶段放热共同作用的结果。4号样品放热峰值和放热速率最大,是由于4号样c(Mn2+)浓度最大,反应放热效应大于Ostwald熟化的吸热效应。
3.3. 反应物离子浓度比对产物形貌尺寸及热动力学行为的影响
实验参数RM的改变对纳米MnMoO4的形貌尺寸的影响结果表6所示。
图6(a)~(d)为样品1~4的SEM图。ω值为20,c为0.05 mol/L–1的条件下,RM比值为1:1和1:2所得

Table 5. Thermodynamic parameters of in situ growth process of MnMoO4 nanostructures
表5. MnMoO4纳米材料原位生长过程的热动力学参数

Table 6. Detailed reaction conditions and products
表6. 详细的反应条件及产物
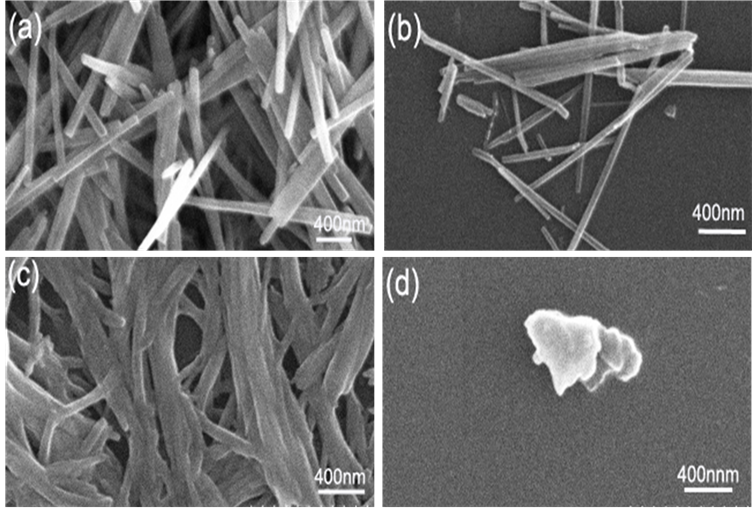
Figure 6. a~d are the SEM patterns of sample 1~4, respectively
图6. (a)~ (d)分别为1~4号样品的SEM图

Table 7. Thermodynamic parameters of in situ growth process of MnMoO4 nanostructures
表7. MnMoO4纳米材料原位生长过程的热动力学参数
的形貌为棒状,当RM比值1:4和1:8时为不规则形貌。
RM比值为1:1比1:2产物尺寸减小,这是因为反应物离子浓度比增大使胶束中粒子总数目增加,增加了碰撞的概率,有利于纳米材料粒径变小。该过程的原位热谱曲线和热动力学参数分别为图6和表7所示。
4. 结论
本文利用高精度、高灵敏度RD496-III型微量热技术获取了不同生长参数下纳米MnMoO4生长的原位热谱信息,结合热动力学理论计算了不同参数下原位生长的热动力学参数,讨论了热动力学参数对其形貌和尺寸的影响机理。获取了以下结论:1) 在双微乳体系中,通过调节不同的生长参数(ω, c, RM)不同形貌尺寸的纳米MnMoO4生长过程的原位热谱曲线;2) 讨论了不同生长参数对纳米MnMoO4原位生长过程的热动力学参数的影响规律,并讨论了生长过程的热动力学参数对形貌尺寸的影响;3) 在双微乳体系中纳米MnMoO4在不同生长参数下原位生长过程的热谱曲线具有相同的热变趋势:均经历了一个快速的放热–吸热–放热过程。整个生长过程分为三个阶段:反应成核阶段、结晶形成阶段、晶体生长阶段,且反应成核阶段的反应吉布斯自由能与反应速率常数成反比。本研究提供从热动力学的角度认识纳米材料原位生长过程的热动力学机理,为指导合成满足特定需求的纳米材料具有重要的科学意义。
基金项目
国家自然科学基金(20963001, 21273050, 21573048)资助项目,广西民族大学2016年研究生教育创新计划项目(gxun-chxzs2016114)资助。