1. 引言
近年来,半导体金属硫化物因其特殊的结构和优异的物理化学性质备受关注,诸如CuS [1] 、ZnS [2] 和CdS [3] 等。其中,窄带隙半导体CuS (Eg: 1.2~2.0 eV)作为一种重要的金属硫化物,在催化 [4] 、纳米开关 [5] 和储能 [6] 等领域获得了广泛关注,成为了研究热点。CuS较窄的带隙以及可见光下显著的光谱响应,使其在光催化降解污染物领域成为新宠。纳米光催化剂的光催化降解效率与其形貌、尺寸和比表面积等密切相关。为了获得不同形貌的硫化铜纳米材料,科研人员开发了多种制备方法,比如,水热法 [7] 、化学沉淀法 [8] 和电化学沉积法 [9] 等。其中,水热制备方法具有结晶度高、操作简便、分散性好等优点。王杰 [10] 等人报道的CuS纳米棒和微米球对亚甲基蓝具有一定的光催化效果。景志红课题组 [11] 以聚乙二醇为表面活性剂,以氯化铜和硫代乙酰胺为铜源、硫源,在160℃下水热反应制备得到管状立方相CuS。该样品对罗丹明和甲基橙具有较好的紫外光光催化效果。因此,探索CuS的制备方法,并将其应用于光催化领域的研究,是我们研究的热点。
本文以硫酸铜和硫脲为原料,一水柠檬酸作为表面活性剂,通过一步简单的水热法制备了CuS纳米粒子,并以甲基橙为目标污染物,分别在可见光和紫外光照射下考察了CuS纳米粒子的光催化性能。
2. 实验
2.1. 试剂
一水硫酸铜(CuSO4∙H2O)、硫脲(CH4N2S)、氢氧化钠(NaOH)和一水合柠檬酸(C6H8O7∙H2O)购买于国药集团化学试剂有限公司。所有药品均为分析纯。
2.2. 实验过程
分别称取三组原料,每组质量为86.00 mg的五水硫酸铜,39.60 mg的硫脲和1.12 mg的氢氧化钠,每组加入质量不同的一水合柠檬酸,分别是157.60 mg、105.10 mg、52.50 mg。然后分别加入到容量为100 mL反应釜中,加入50 mL去离子水。常温下搅拌30 min使其混合均匀,出现浑浊现象。将所得液体在160℃条件下水热反应10 h。自然冷却后,取其上层清液,离心取出沉淀,用水和酒精分别洗若干遍,并干燥,得到产物。
2.3. 分析表征
利用X射线衍射仪(PANalytical B.V. X’Pert PRO)和扫描电子显微镜(Nova NanoSEM 450)表征材料的形貌和结构。
2.4. 光催化活性测定
称取50 mg制备的CuS粉末加入到50 mL质量浓度为10 mg/L甲基橙溶液中,在黑暗环境中搅拌使样品与溶液达到吸脱附平衡。然后在350 W金卤灯照射下模拟可见光,在400 W汞灯照射下模拟紫外光。每间隔10 min取样,8000 rpm离心取上清液。接着用紫外可见分光光度计(UH5300,Hitachi)测定吸光度并分析光催化性能。
3. 结果和讨论
CuS的光催化降解有机污染物机理可用图1进行说明:经光照后,CuS纳米粒子内部产生光生电子–空穴对,当电子和空穴分别迁移到颗粒表面时,光生电子与O2结合产生超氧自由基(O*2),而光生空穴与H2O产生羟基自由基(*OH)。超氧自由基和羟基自由基具有很强的氧化还原能力,可以将污染物矿化降解成无害物质。
图2为不同柠檬酸浓度制备的CuS样品的XRD图。图中的8个峰值,27.608˚、29.104˚、31.613˚、32.620˚、38.844˚、47.974˚、52.654˚和59.221˚,分别与标准卡片(JCPDS No. 06-0464)中的晶面(101)、(102)、(103)、(006)、(105)、(110)、(108)和(116)相吻合,这表明合成的样品为六方相CuS。同时在XRD图谱中没有发现其他物相的杂峰,这说明制备的样品为CuS。
图3为不同柠檬酸浓度条件下合成CuS的SEM图,可见,水热反应得到了形貌不规则的CuS纳米粒子。通过图中对比可发现,水热反应过程中,当柠檬酸浓度较低,为1.05 g/L时,产物CuS颗粒尺寸较大,且团聚现象严重。当柠檬酸的浓度增加到2.10 g/L时,CuS颗粒尺寸减小,聚集性降低,分散性变好。继续增加柠檬酸浓度到3.15 g/L,所得产物的尺寸与分散性与柠檬酸浓度为2.10 g/L时差别不大。我们推测,出现这种现象的原因主要是:在水热反应阶段,硫脲首先发生水解。
生成的H2S与溶液中的Cu2+发生反应生成CuS。
表面活性剂柠檬酸在反应中会与金属阳离子产生比较强的螯合作用,柠檬酸中的羧基可以紧密地吸附在CuS表面 [12] ,从而抑制了CuS晶核的生长速度。因此,随着柠檬酸浓度的增加,CuS颗粒尺寸减小,分散性变好。而当柠檬酸浓度过高时,在CuS晶核生长阶段,其表面吸附的柠檬酸的量已到达饱和,故继续提高柠檬酸的量,对其尺寸和分散性的改变影响不大。
图4为不同柠檬酸浓度合成CuS在紫外和可见光源照射下对有机污染物甲基橙的光催化降解曲线图。图中实线为CuS在可见光照射下光催化降解甲基橙曲线,虚线为CuS在紫外光照射下光催化降解甲基橙曲线,黑色曲线为没有添加CuS的甲基橙溶液在紫外/可见光照射下的降解曲线。实验发现CuS不仅在紫外光照射下具有光催化能力,而且在可见光照射下也具有光催化能力。表明CuS是一种潜在的可见光光催化材料。当柠檬酸浓度为1.05 g/L时,CuS在两种不同光源照射下的光催化降解甲基橙曲线基本相同,在20分钟内可以将甲基橙溶液降解一半左右。当柠檬酸浓度为2.10 g/L和3.15 g/L时,所得的

Figure 1. The principle diagram of photocatalysis by CuS
图1. CuS光催化原理图

Figure 2. XRD patterns for CuS using different citric acid concentration
图2. 不同柠檬酸浓度制备的CuS的XRD图
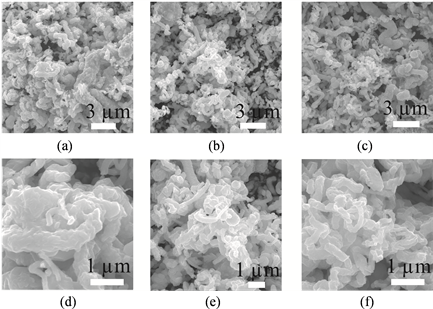
Figure 3. The scanning electron microscope (SEM) images of CuS using different citric acid concentration (a and d) 1.05 g/L, (b and e) 2.10 g/L, (c and f) 3.15 g/L
图3. 使用不同浓度柠檬酸合成CuS的扫描电子显微镜(SEM)图:柠檬酸浓度(a和d)为1.05 g/L,(b和e)为2.10 g/L,(c和f)为3.15 g/L

Figure 4. The photocatalytic properties of CuS prepared by different citric acid concentration under Ultraviolet/Visible light irradiation
图4. 不同柠檬酸浓度合成CuS在紫外/可见光照射下的光催化性能
CuS纳米粒子可以在10分钟左右将甲基橙溶液降解一半左右。出现这种现象的原因可能是:与低柠檬酸浓度制备出的CuS样品相比,在柠檬酸浓度为2.10 g/L和3.15 g/L的条件下制备出来的CuS纳米粒子具有更小的尺寸和更好的分散性,有利于光催化过程中染料分子的吸附,因此表现出更优的光催化性能。
4. 结论
本文主要采用简易水热法,利用柠檬酸作为表面活性剂,制备了CuS纳米粒子。通过XRD表征证明产物为六方相纯CuS。SEM结果表明,柠檬酸浓度对CuS的尺寸和分散性有显著影响,低浓度柠檬酸条件水热合成的CuS容易团聚且尺寸较大,高浓度柠檬酸条件下合成的CuS尺寸较小且分散性较好,当柠檬酸浓度达到一定值时,继续增加柠檬酸浓度对产物尺寸的变化影响不大。光催化降解甲基橙结果表明,CuS纳米粒子同时具有紫外和可见光催化活性。高柠檬酸浓度制备出的CuS样品表现出更优的光催化性能。
致谢
这项工作是由湖北省自然科学基金项目(2014CFB631, 2015CFB498),湖北省教育厅科研计划(Q20141802, Q20111801)和湖北汽车工业学院博士启动资金(BK201301, BK201503)支持。本工作受光电子技术湖北省协同创新中心专项经费资助。