1. 引言
砜基化合物具有广谱生物活性,包括抗菌、抗炎和抗肿瘤等 [1]。此外,砜基化合物还被广泛用于除虫、除草等农药应用领域 [2]。β-羰基砜是一类重要的有机合成砌块,被广泛用于制备不饱和化合物炔烃和烯烃、多官能化的4H-吡喃化合物和光学活性的β-羟基砜等产物 [3] [4] [5] [6]。因此,β-羰基砜类化合物的合成备受关注,常见的合成方法为采用磺酰自由基和烯烃的分子间加成来实现。磺酰自由基的来源包括亚砜、亚磺酸、磺酰肼、磺酰氯化物和磺酰叠氮化合物等 [7] - [15]。最近,以烯醇硅醚为自由基受体的磺酰自由基加成反应也被应用于β-羰基砜化合物的合成 [16]。然而,这些方法往往存在反应条件比较苛刻、产率不够高以及存在一些副反应等不足。因此,发展反应条件温和、操作更加简便和更高选择性的绿色合成方法具有十分重要的意义。
电化学催化法用电子作为氧化还原试剂,避免使用金属催化剂,减少了合成反应后处理带来的环境污染等问题,符合绿色化学发展的需要,受到广泛的关注 [17] [18]。至今,电化学催化法已被广泛应用于C-C、C-O、C-N和C-S键的构建。例如,通过电化学氧化磺酰肼的N-S键断裂生成磺酰自由基,黄精美教授课题组成功构建了C-S键。
在前期研究工作的基础上,本文以四丁基碘化铵为催化剂,采用电化学催化法,使磺酰肼与烯醇硅醚发生自由基偶联反应,在室温条件下合成了一系列的β-羰基砜化合物。该反应具有反应条件温和、操作简便和避免使用有毒催化剂等优点,更加绿色环保。
2. 实验部分
2.1. 主要仪器与试剂
Bruker-400型核磁共振仪(CDCl3为溶剂,TMS为内标)、BrukermicroTOF-Q II型高分辨质谱仪(国布鲁克公司),电化学合成仪ElectraSyn2.0 (德国IKA)。
柱分离用200~300目硅胶、溶剂和试剂均为市售分析纯;乙腈(分析纯,南京化学试剂股份有限公司);试验所用苯乙酮、碘化钠、三乙胺、三甲基氯硅烷、磺酰肼和四丁基碘化铵均购自北京伊诺凯科技有限公司。
2.2. 实验方法
烯醇硅醚类化合物的合成:在50 mL充满氩气的三口烧瓶中加入10 mmol苯乙酮类化合物、15 mmol (4.5 g)碘化钠和10 mL干燥的乙腈,室温下搅拌反应5 min;加入15 mmol (4.2 mL)三乙胺和15 mmol (3.82 mL)三甲基氯硅烷,于40℃搅拌反应12 h;用薄层色谱法(TLC)监测反应到完全后,加入50 mL冰水淬灭反应。随后加入正戊烷(15 mL × 3)进行萃取;合并有机相,用饱和食盐水洗涤、硫酸镁干燥,经柱层析分离(石油醚/乙酸乙酯体积比30:1),得到烯醇硅醚类化合物。
β-羰基砜类化合物的合成:于25 mL的三颈圆底烧瓶中加入38.5 mg烯醇硅醚1 (0.2 mmol),37.2 mg磺酰肼2 (0.2 mmol),36.9 mg四丁基碘化铵(0.1 mmol),15 ml乙腈,混合均匀,15 mm * 15 mm铂片为正极和负极,室温下以9 mA电流通电5小时。反应完全后,混合物减压脱溶,得残余物,柱层析(石油醚/乙酸乙酯体积比60:1)纯化得到产物。
1-phenyl-2-tosylethan-1-one (3aa): white solid; 1H NMR (400 MHz, CDCl3) δ: 7.95 (d, J = 7.6 Hz, 2H), 7.74 (d, J = 8.0 Hz, 2H), 7.60 (t, J = 7.6 Hz, 1H), 7.44 (t, J = 8.0 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 4.70 (s, 2H), 2.41 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 188.0, 145.1, 135.5, 135.4, 134.1, 129.5, 129.1, 128.6, 128.4, 63.4, 21.4; HRMS m/z (ESI) calcd for C15H15O3S (M+H)+ 275.3414, found 275.3423.
1-(p-tolyl)-2-tosylethan-1-one (3ab): white solid; 1H NMR (400 MHz, CDCl3) δ: 7.84 (d, J = 8.0 Hz, 2H), 7.73 (d, J = 8.0 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 8.4 Hz, 2H), 4.66 (s, 2H), 2.43 (s, 3H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3) δ:187.2, 145.2, 145.0, 135.5, 133.0, 129.4, 129.2, 129.1, 128.2, 63.2, 21.4, 21.2; HRMS m/z (ESI) calcd for C16H17O3S (M+H)+ 289.3684, found 289.3692.
1-(4-fluorophenyl)-2-tosylethan-1-one (3ac): white solid; 1H NMR (400 MHz, CDCl3) δ: 8.01-7.97 (m, 2H), 7.73 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 7.17-7.11 (m, 2H), 4.65 (s, 2H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 186.3, 166.2 (d, J = 256.2 Hz), 145.3, 135.4, 132.0 (d, J = 2.4 Hz), 131.9, 129.6, 128.3, 116.0(d,J =22.4 Hz), 63.5, 21.4; HRMS m/z (ESI) calcd for C15H14O3S (M+H)+ 293.0642, found 293.0638.
1-(naphthalen-2-yl)-2-tosylethan-1-one (3ad): white solid; 1H NMR (400 MHz, CDCl3) δ: 8.40 (s, 1H), 8.00-7.92 (m, 2H), 7.88-7.81 (m, 2H), 7.74 (d, J = 8.0 Hz, 2H), 7.62-7.48 (m, 2H), 7.25 (d, J = 7.6 Hz, 2H), 4.81 (s, 2H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 188.0, 145.1, 135.5, 135.3, 133.0, 132.0, 132.0, 129.7, 129.5, 129.0, 128.5, 128.2, 127.4, 127.0, 123.6, 63.4, 21.4; HRMS m/z (ESI) calcd for C19H17O3S (M+H)+325.0893, found 325.0884.
1-m-tolyl-2-tosylethanone (3af): white solid;1H NMR (400 MHz, CDCl3) δ: 7.75-7.73 (m, 2H), 7.71-7.65 (m, 2H), 7.38 (d, J = 7.6 Hz, 1H), 7.31 (dd, J = 17.5, 7.6 Hz, 3H), 4. 68 (s, 2H), 2. 41 (s, 3H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 188.0, 145.1, 138.6, 135.6, 135.6, 135.0, 129.5, 129.3, 128.5, 128.4, 126.4, 63.3, 21.5, 21.1; HRMS m/z (ESI) calcd for C16H17O3S (M+H)+ 289.0892, found 289.0883.
2-((4-methoxyphenyl)sulfonyl)-1-phenylethan-1-one (3ba): white solid; 1H NMR (400 MHz, CDCl3) δ: 7.94 (dd, J = 8.0, 1.1 Hz, 2H), 7.81-7.75 (m, 2H), 7.63-7.56 (m, 1H), 7.50-7.45 (m, 2H), 7.00-6.92 (m, 2H), 4.68 (s, 2H), 3.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 187.5, 163.4, 135.1, 133.5, 130.1, 129.5, 128.6, 128.1, 114.0, 63.2, 55.3; HRMS m/z (ESI) calcd for C15H15O4S (M+H)+ 291.0685, found 291.0693.
1-phenyl-2-((4-(trifluoromethyl)phenyl)sulfonyl)ethan-1-one (3bb): white solid; 1H NMR (400 MHz, CDCl3) δ: 8.03 (d, J = 8.0 Hz, 2H), 7.90 (dd, J = 8.0, 1.2 Hz, 2H), 7.80 (d, J = 8.0 Hz, 2H), 7.65-7.60 (m, 1H), 7.46 (t, J = 8.0 Hz, 2H), 4.76 (s, 2H); 13C NMR (100 MHz, CDCl3) δ:187.3, 141.5, 135.5, 135.0 (d, J = 15.8Hz), 134.0 129.0 129.0, 128.6 126.6 (q, J = 3.0 Hz), 123.0 (q, J = 268.0 Hz), 62.6; HRMS m/z (ESI) calcd for C15H12F3O3S (M+H)+ 329.0453, found 329.0460.
1-phenyl-2-(m-tolylsulfonyl)ethanone (3bc): white solid; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 7.6 Hz, 2H), 7.67 (s, 2H), 7.60 (t, J= 7.6 Hz, 1H), 7.45-7.41 (m, 4H), 4.71 (s, 2H), 2.42 (s, 3H);13C NMR (100 MHz, CDCl3) δ: 188.1, 139.5, 138.4,135.6, 135.1, 134.1, 129.0, 129.0, 128.7, 128.6, 125.5, 63.5, 21.2; HRMS m/z (ESI) calcd for C15H15O3S (M+H)+ 275.0736, found 275.0732.
3. 结果与讨论
3.1. 反应条件的筛选
以烯醇硅醚1a (0.2 mmol)和磺酰肼2a (0.2 mmol)为底物,以四丁基碘化铵(0.1 mmol)为催化媒介的反应为模型,我们探讨了催化媒介的类型、溶剂的种类、电流的大小以及反应温度对模型反应产率的影响(表1)。
首先探讨催化媒介对反应的影响(表1,entries 1~4)。从表中可以看出,无催化媒介条件下不能发生反应;使用碘化铵、溴化铵均不如四丁基碘化铵的产率高;增加催化媒介的量不能提高反应产率。对溶剂进行了筛选,发现溶剂甲醇、乙醇和DMSO均会使产率有所下降(表1,entries 5~7),这可能与碘化铵的溶解度有关。随后对电流进行筛选,研究表明没有电流时,反应不能发生;电流为8 mA和12 mA时,产率均有所下降,说明10 mA电流为最合适的电流(表1,entries 8~10)。最后,对反应温度进行了考察(表1,entries 11~13),结果表明提高或降低反应温度不能使产率提高。因此,此反应的最优反应条件为:以四丁基碘化铵为媒介、乙腈为溶剂、10 mA电流,室温下反应5小时。

Table 1. Optimization of reaction conditions
表1. 反应条件的优化
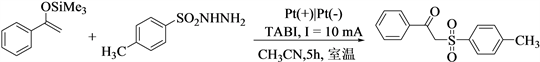
3.2. 目标产物的普适性研究
根据上述建立的最优反应条件(表1,entries 2),得到了中等产率的目标化合物。我们对该反应的底物普适性进行了研究,结果如表2所示。从表中可以看出,烯醇硅醚的苯环对位上无论带有强吸电子基团还是给电子基团,都能很好的发生反应,以中等产率得到目标化合物(表2,entries 1~3);而在邻位有给电子基团时,也能很好的发生反应(表2,entries 4);但在邻位有吸电子基团时,反应难以发生(表2,entries 5)。此外,在磺酰肼的苯环对位无论是吸电子基还是给电子基,反应均能较好的发生(表2,entries 7~8)。同时,在间位为甲基时,以中等产率得到目标产物(表2,entries 6, 9)。因此,该反应体系具有较好的底物普适性。

Table 2. Investigation of substrate scope
表2. 底物普适性研究

3.3. 反应机理分析
以模型反应为研究对象,在最优反应条件下对反应机理进行探讨。当反应中加入0.2 mmol自由基抑制剂2, 2, 6, 6-四甲基哌啶氮氧化物时,反应受到抑制。参考文献16,提出如下图1所示的反应机理:电化学条件下,碘负离子在阳极被氧化成碘自由基,该自由基进攻磺酰肼,导致磺酰肼脱氢,并进一步脱氮气,生成磺酰自由基。然后,磺酰自由基进攻烯基硅醇的末羰双键,生成自由基中间体,经单电子转移并脱氢和脱三甲基碘硅烷后得到目标化合物3。
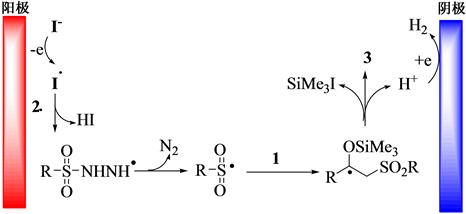
Figure 1. Plausible mechanism for synthesis of compound 3
图1. 合成化合物3可能的反应机理
4. 结论
采用碘盐媒介的电化学催化磺酰肼活化得到磺酰基,在室温下实现了烯醇硅醚和磺酰肼的自由基偶联反应合成β-羰基砜化合物。该反应条件简单,底物的普适性较好,在温和的条件下以中等收率获得了一系列的β-羰基砜化合物。该反应避免使用金属、碘等,且副产物为氮气和氢气,绿色环保。
NOTES
*通讯作者。