摘要: 目的:探究新型TK抑制剂达克替尼对人肺腺癌A549细胞增殖与侵袭作用及机制。方法:人肺腺癌A549细胞为研究对象,根据预实验结果,将人肺腺癌细胞系A549分成3组,即空白对照组,低剂量组和高剂量组。RT-PCR检测EFGR和凋亡蛋白Caspase-3和Caspase-9的表达,流式细胞仪检测细胞的分裂周期和细胞凋亡率,CCK-8检测细胞的增殖。结果:空白对照组,低剂量组和高剂量组的EFGR,Caspase-3和Caspase-9 mRNA相对表达量相对量比较具有统计学差异(P < 0.05);空白对照组的EFGR mRNA相对表达量相对量要明显高于低剂量组和高剂量组(P < 0.05),空白对照组的Caspase-3和Caspase-9 mRNA相对表达量要明显低于低剂量组和高剂量组(P < 0.05),低剂量组和高剂量组的EFGR,Caspase-3和Caspase-9 mRNA相对表达量相对量比较无统计学差异(P > 0.05)。低剂量组和高剂量组的G0/G1期比例明显高于空白对照组(P < 0.05);S期和G2期比例明显低于空白对照组(P < 0.05)。低剂量组和高剂量组的总凋亡率明显高于空白对照组(P < 0.05);高剂量组晚期凋亡率明显高于低剂量组(P < 0.05)。药物处理2天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05)。结论:低剂量达克替尼可以抑制肺腺癌细胞的增殖,促进肺腺癌细胞的凋亡,并且可以抑制EFGR的表达,可以作为耐药性肺腺癌的治疗方案。
Abstract:
Objective: The objective is to investigate the proliferation and invasion of human lung adenocar-cinoma A549 cells induced by a novel TK inhibitor, dacomitinib, and its mechanism. Methods: Human lung adenocarcinoma cell line A549 was divided into three groups according to the pre-liminary results, namely the blank control group, the low-dose group and the high-dose group. The expression of EFGR and Caspase-3 and Caspase-9 was detected by RT-PCR. The cycle of cell division and apoptotic rate of the cell were detected by flow cytometry. The cell proliferation was detected by CCK-8. Results: The relative expressions of EFGR, caspase-3 and caspase-9 mRNA in blank control group, low-dose group and high-dose group were statistically different (P < 0.05). The relative expression of EFGR mRNA in the blank control group was significantly higher than that in the low-dose group and the high-dose group (P < 0.05). The relative expression of Caspase-3 and caspase-9 mRNA in the blank control group was significantly lower than that in the low-dose group and high-dose group (P < 0.05). There was no significant difference in the relative expression of EFGR, caspase-3 and caspase-9 mRNA between the low-dose group and the high-dose group (P > 0.05). The proportions of G0/G1 phase in low-dose group and high-dose group were significantly higher than those in blank control group (P < 0.05), while the proportions of S phase and G2 phase were significantly lower than those in blank control group (P < 0.05). The total apoptotic rate of the low-dose group and the high-dose group significantly higher than that of the blank control group (P < 0.05), and the late apoptotic rate of the high-dose group was significantly higher than that of the low-dose group (P < 0.05). After 2 days of drug treatment, the cell increment rate of blank control group was significantly higher than that of low-dose group and high-dose group (P < 0.05); after 3 days of drug treatment, the cell increment rate of blank control group was significantly higher than that of low-dose group and high-dose group (P < 0.05). Conclusion: Low-dose of dacomitinib can inhibit the proliferation of lung adenocarcinoma cells, promote the apoptosis of lung adenocarcinoma cells, and inhibit the expression of EFGR. It can be used as a therapeutic regimen for drug-resistant lung adenocarcinoma.Objective: The objective is to investigate the proliferation and invasion of human lung adenocarci-noma A549 cells induced by a novel TK inhibitor, dacomitinib, and its mechanism. Methods: Human lung adenocarcinoma cell line A549 was divided into three groups according to the preliminary re-sults, namely the blank control group, the low-dose group and the high-dose group. The expression of EFGR and Caspase-3 and Caspase-9 was detected by RT-PCR. The cycle of cell division and apop-totic rate of the cell were detected by flow cytometry. The cell proliferation was detected by CCK-8. Results: The relative expressions of EFGR, caspase-3 and caspase-9 mRNA in blank control group, low-dose group and high-dose group were statistically different (P < 0.05). The relative expression of EFGR mRNA in the blank control group was significantly higher than that in the low-dose group and the high-dose group (P < 0.05). The relative expression of Caspase-3 and caspase-9 mRNA in the blank control group was significantly lower than that in the low-dose group and high-dose group (P < 0.05). There was no significant difference in the relative expression of EFGR, caspase-3 and caspa-se-9 mRNA between the low-dose group and the high-dose group (P > 0.05). The proportions of G0/G1 phase in low-dose group and high-dose group were significantly higher than those in blank control group (P < 0.05), while the proportions of S phase and G2 phase were significantly lower than those in blank control group (P < 0.05). The total apoptotic rate of the low-dose group and the high-dose group significantly higher than that of the blank control group (P < 0.05), and the late apoptotic rate of the high-dose group was significantly higher than that of the low-dose group (P < 0.05). After 2 days of drug treatment, the cell increment rate of blank control group was signifi-cantly higher than that of low-dose group and high-dose group (P < 0.05); after 3 days of drug treatment, the cell increment rate of blank control group was significantly higher than that of low-dose group and high-dose group (P < 0.05). Conclusion: Low-dose of dacomitinib can inhibit the proliferation of lung adenocarcinoma cells, promote the apoptosis of lung adenocarcinoma cells, and inhibit the expression of EFGR. It can be used as a therapeutic regimen for drug-resistant lung ade-nocarcinoma.
1. 引言
肺癌是临床上的多发病,尤其是随着生活环境的改变,生活压力的激增,人们饮食习惯的改变,肺癌的发病率明显升高。据流行病学统计结果显示,全球每年新增的肺癌患者约几万例,多见于发展中国家 [1] [2] [3]。目前对于肺癌患者的治疗,化疗方案是较为理想的治疗方案。有研究表明,表皮生长因子受体酪氨酸激酶抑制剂(Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors, EGFR-TKIs)是比较有效的治疗方案之一,比如吉非替尼等 [4],但是,该类治疗方案也存在一定的问题,比如药物耐药性的问题 [5]。并且与表皮生长因子(EFGR)的表达量相关,为了解决这个问题,第二代TKI应运而生,代表药物是达克替尼。有研究表明,达克替尼可以抑制EFGR的表达,从而达到抑制耐药性的作用 [6]。但是,关于达克替尼的细胞学实验较少,因此,本研究以人肺腺癌A549细胞为研究对象,验证达克替尼的生物学行为,以期为新型TK抑制剂达克替尼治疗人肺腺癌提供理论依据。
2. 材料与方法
2.1. 肺腺癌细胞系A549的培养
细胞系购自北京索莱宝生物有限公司,均经过str检测,证明细胞没有被污染。
2.2. 实验分组
根据预实验结果,将人肺腺癌细胞系A549分成3组,即空白对照组,低剂量组和高剂量组。低剂量组细胞给予1 μmol/L的达克替尼进行处理3 h;高剂量组细胞给予10 μmol/L的达克替尼进行处理3 h;空白对照组细胞给予等计量的PBS溶液进行对照处理。
2.3. 实时荧光定量PCR检测Akt1、Caspase-3和Caspase-9 mRNA的表达水平
收集上述3组细胞,加入1 mL Trizol试剂,充分混合后,提取细胞总RNA,室温条件下根据逆转录试剂盒说明书,将总RNA逆转录为cDNA,−20℃低温冰箱保存。然后根据荧光定量PCR试剂盒说明书,加入cDNA和USP-22基因和ERK5的引物模板(如表1所示),置于Applied Biosystems PCR仪进行反应,设置条件按照说明书。统计并记录各样本CT值。

Table 1. The primer sequence of target gene
表1. 目的基因的引物序列
2.4. 细胞凋亡水平
利用流式细胞仪进行检测,加入AV-PI试剂盒的混合制剂,然后流式细胞仪上机测试,验证细胞的凋亡水平。
2.5. 细胞增殖能力
利用CCK-8试剂盒检测细胞增殖能力,加入CCK-8试剂盒的混合制剂,利用酶标仪进行测定,波长为450 nm。
2.6. 统计学方法
计数资料用
± s形式表示,SPSS 20.0软件进行统计学分析,3组的RT-PCR数据结果,细胞增值率和细胞凋亡率比较采用单因素方差分析,组间的比较采用q检验,认为P < 0.05有统计学意义。
3. 结果
3.1. EFGR,Caspase-3和Caspase-9的mRNA相对表达量
结果表明,空白对照组,低剂量组和高剂量组的EFGR,Caspase-3和Caspase-9的mRNA相对表达量比较具有统计学差异(F = 10.209, 9.892, 11.092; P < 0.05);其中低剂量组和高剂量组的EFGR的mRNA相对表达量要明显低于空白对照组(P < 0.05),Caspase-3和Caspase-9的mRNA相对表达量要明显高于空白对照组(P < 0.05) (见图1)。

Figure 1. The relative mRNA expression of EFGR, caspase-3 and caspase-9. Note: ** indicates statistical difference (P < 0.05); # indicates no statistical difference (P > 0.05)
图1. EFGR,Caspase-3和Caspase-9的mRNA相对表达量。注:**表示具有统计学差异(P < 0.05);#表示没有统计学差异(P > 0.05)
3.2. 3组细胞周期比较结果
结果表明,低剂量组和高剂量组的G0/G1期比例明显高于空白对照组(P < 0.05);S期和G2期比例明显低于空白对照组(P < 0.05) (见图2)。
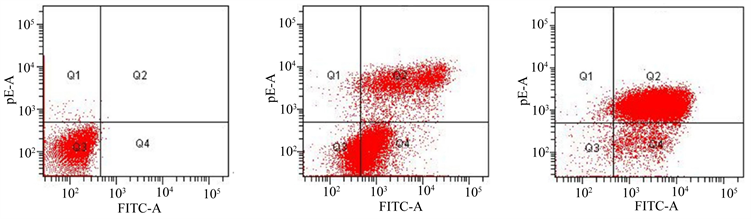
3.3. 3组细胞凋亡率比较结果
结果表明,低剂量组和高剂量组的总凋亡率明显高于空白对照组(P < 0.05);高剂量组晚期凋亡率明显高于低剂量组(P < 0.05) (见图3)。
3.4. 3组细胞增殖率比较结果
结果显示,药物处理2天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(q = 2.293, 2.775; P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(q = 2.887, 2.461; P < 0.05) (见图4)。
 (a) (b)(c)
(a) (b)(c)
Figure 2. The comparison of cell cycle among three groups. (a) The blank control group; (b) The low-dose group; (c) The high-dose group
图2. 3组细胞周期比较结果。(A) 空白对照组;(B) 低剂量组;(C) 高剂量组
 (a) (b)(c)
(a) (b)(c)
Figure 3. The comparison of apoptosis rate among three groups. (a) The blank control group; (b) The low-dose group; (c) The high-dose group
图3. 3组细胞凋亡率比较结果。(a) 空白对照组;(b) 低剂量组;(c) 高剂量组
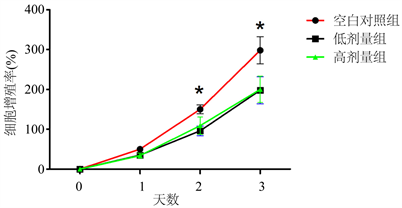
Figure 4. The comparison of proliferation rate among three groups. Note: * indicates statistical difference (P < 0.05)
图4. 3组细胞增值率比较结果。注:*表示具有统计学差异(P < 0.05)
4. 讨论
本研究结果表明,根据预实验结果给予1 μmol/L低剂量的达克替尼和10 μmol/L高剂量的达克替尼两种浓度进行处理,并且加入等量PBS溶液进行对比研究。RT-PCR检测EFGR和凋亡蛋白Caspase-3和Caspase-9的表达。流式细胞仪检测细胞的分裂周期和细胞凋亡率,CCK-8检测细胞的增殖。结果表明,达克替尼处理人肺腺癌A549细胞,可以明显降低EFGR mRNA相对表达量和蛋白表达量,增加Caspase-3和Caspase-9 mRNA相对表达量和蛋白表达量。并且,低剂量组和高剂量组的G0/G1期比例明显高于空白对照组(P < 0.05);低剂量组和高剂量组的总凋亡率明显高于空白对照组(P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05)。说明,达克替尼可以抑制细胞增殖,促进细胞凋亡。可以作为EFGR阳性的肺腺癌A549细胞的治疗药物。
对于肺腺癌患者而言,尤其是晚期肺腺癌,表皮生长因子受体(Epidermal Growth Factor Receptor, EFGR)突变是其耐药性的主要特点 [7] [8] [9]。周玮玮等人 [10] 利用量子点(QDs)荧光探针检测人肺腺癌组织,结果表明,晚期肺腺癌组织可见EFGR的高表达。史张等人 [11] 对581例肺腺癌患者进行检测,可见132例EFGR的高突变,结果表明,EFGR的高突变与肺腺癌的增殖和侵袭有关。本研究在前人的基础上,利用第二代表皮生长因子受体酪氨酸激酶抑制剂(Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors, EGFR-TKIs)达克替尼进行治疗,结果表明,达克替尼可以抑制EFGR的mRNA相对表达水平和蛋白表达水平,说明达克替尼可以抑制EFGR的表达。
既往研究表明,Caspase-3和Caspase-9是与细胞凋亡密切相关,因此,也有学者称他们为“凋亡相关蛋白”,将Caspase-3和Caspase-9作为细胞凋亡的指示分子 [12] [13] [14]。本研究以Caspase-3作为凋亡的靶点,结果表明,达克替尼可以促进Caspase-3的mRNA相对表达水平和蛋白表达水平,说明达克替尼可以促进肺腺癌细胞的凋亡。另外,本研究利用CCK-8试剂盒检测细胞的增殖,结果表明,药物处理2天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05);药物处理3天后,空白对照组的细胞增值率要明显高于低剂量组和高剂量组(P < 0.05)。结果说明,达克替尼可以抑制肺腺癌细胞的增殖。并且与达克替尼的剂量无关。
综上所述,低剂量达克替尼可以抑制肺腺癌细胞的增殖,促进肺腺癌细胞的凋亡,并且可以抑制EFGR的表达,可以作为耐药性肺腺癌的治疗方案。
参考文献