摘要: 目的:明确肝内胆管细胞癌合并淋巴结转移的患者行淋巴结清扫术的安全性以及可能获益。方法:分析1例原发性肝内胆管细胞癌合并巨大的肝门部淋巴结转移的中老年男性患者的临床表现、影像学表现、手术情况、术后并发症以及随访情况。结果:此例患者手术时间为315分钟,术中出血500 ml,术后住院时间8天,无术后主要并发症,该患者病理为中–低分化胆管细胞癌,肿瘤大小5 × 3.5 × 3 cm,8、12、13组淋巴结转移癌并融合肿块,目前随访8个月未见肿瘤复发。结论:肝内胆管细胞癌合并淋巴结转移的患者行淋巴结清扫术,手术风险可控,可能延长患者的无病生存期。
Abstract:
Objective: To determine the safety and potential benefit of lymph node dissection in patients with intrahepatic cholangiocellular carcinoma with lymph node metastasis. Methods: A case of primary intrahepatic cholangiocarcinoma with large hilar lymph node metastasis was analyzed, including the clinical manifestations, imaging manifestations, surgical status, postoperative complications and follow-up. Results: The operation time of this patient was 315 minutes, the intraoperative hemorrhage was 500 ml, the postoperative hospital stay was 8 days, and no major postoperative complications were observed. The patient was pathologically identified as moderately to poorly differentiated cholangiocarcinoma with tumor size of 5 × 3.5 × 3 cm, lymph node metastasis and mass fusion in group 8, 12, and 13, and no tumor recurrence was observed during 8 months of follow-up. Conclusion: Lymph node dissection in patients with intrahepatic cholangiocellular carcinoma with lymph node metastasis can control the risk of operation and may prolong the disease-free survival of patients.
1. 前言
肝内胆管细胞癌(Intrahepatic Cholangio Carcinoma, ICC)是指发生在包括二级胆管在内的末梢侧胆管上皮细胞恶性肿瘤。外科手术是对ICC患者可能治愈的手段之一,但是否行淋巴结清扫尚存在争议。针对可切除的肝内胆管癌,我们团队常规进行肝门部淋巴结清扫术。在保证患者围手术期安全性的前提下尽可能的使患者获益。我们收治了1例罕见的肝内胆管细胞癌合并肝门巨大淋巴结转移的病例,其特点为肝内病灶大小仅5 × 3.5 × 3 cm,而肝门部却形成多个淋巴结转移癌融合成巨大肿块体积达到9.5 × 5 × 4.5 cm。现对此病例诊疗过程报告并对其手术风险、预后生存期讨论分析如下。
2. 病例资料
患者男性,59岁,主诉“右上腹疼痛1月”于2020年9月5日入院,患者近1月以来反复出现右上腹疼痛,无恶心、呕吐,无畏寒发热,无皮肤巩膜黄染,无腹痛腹泻,无呕血黑便等,既往高血压病史1年,无肝炎肝硬化及血吸虫病史,家族中无遗传病及肿瘤病史。入院时患者精神状态良好,食欲体力正常,大小便正常,睡眠好,体重无明显变化。体格检查:生命体征平稳,皮肤巩膜无黄染,腹部柔软、全腹无压痛、反跳痛,未触及包块。无移动性浊音,肠鸣音正常。
实验室检查:肿瘤指标CEA:0.74 μg/L (正常参考值:0~5.0),AFP:3.21 μg/L (正常参考值:0~20),CA19-9:6.96 U/mL (正常参考值:0.1~37),其余血常规、凝血功能、肝肾功能未见异常。
入院前影像学检查外院MRI (2020-08-03)提示:肝右叶肿瘤,考虑恶性,肝门部淋巴结转移。我院超声引导下肝脏肿瘤穿刺活检术病理(2020-08-19)提示:低分化胆管细胞癌。术前影像:我院MRI (2020-09-10)肝右叶恶性肿瘤,肝门部巨大淋巴结转移(详见图1)。

Figure 1. MRI (2020-09-10) Malignant tumor in the right lobe of liver with large hilar lymph node metastasis
图1. MRI (2020-09-10)肝右叶恶性肿瘤,肝门部巨大淋巴结转移
患者明确诊断为:肝右叶肝内胆管细胞癌伴肝门部巨大淋巴结转移。术前胸片、心电图未见明显手术禁忌。2020年9月14日行肝右叶肿瘤切除、肝门部淋巴结清扫术。手术时长:315分钟,出血500 ml,未输血。手术过程顺利,但异常困难,转移的淋巴结巨大且部位特殊,操作受限。具体操作过程见图2~图4。

Figure 2. (a) A hard mass 10 cm behind the hepatioduodenal ligament, irregular in shape, from the right side of the hilum to the left side, closely related to the duodenal bulb, hepatic artery, portal vein, common bile duct and inferior vena cava, with many peripheral collateral vessels, poorly clear and fixed anatomical structure. (LN: lymph node; PH: hilar); (b): Kocher incision dissociates the descending duodenum to expose inferior vena cava and right renal vein. The tumor was closely adhered to the duodenal bulb. (LN: lymph node; Du: duodenal bulb; IVC: inferior vena cava; RRV: right renal vein); (c): Right to left free mass to left inferior vena cava, revealing abdominal aorta (AA), left renal vein (LRV), identifying common bile duct configuration; (d): The gallbladder triangle was dissected, the gallbladder bed was removed to the neck, and the gallbladder duct and gallbladder artery were removed temporarily. The following hepatoduct to the common bile duct by tumor extrusion, dense adhesion, sharp separation adhesion, tape hanging. The right side of the free bile duct was exposed to the portal vein, and the wall of the duct was squeezed by the mass with sharp separation and adhesion. LN (arrow): lymph node; CBD: common bile duct; PV: portal vein; PaH: head of pancreas)
图2. (a) 肝十二指肠韧带后方10 cm质硬肿块,形态不规则,由肝门右侧,后方至左侧,与十二指肠球部、肝动脉、门静脉、胆总管及下腔静脉关系致密,周围侧枝血管较多,解剖结构欠清,固定。(LN:淋巴结;PH:肝门);(b):Kocher切口游离十二指肠降段,显露下腔静脉,右肾静脉。肿瘤与十二指肠球部黏连致密。(LN:淋巴结;Du:十二指肠球部;IVC:下腔静脉;RRV:右肾静脉);(c):由右至左游离肿块至下腔静脉左侧,显露腹主动脉(AA),左肾静脉(LRV),辨认胆总管走形;(d):解剖胆囊三角,游离胆囊床至颈部,游离胆囊管及胆囊动脉暂不切除。肝总管以下至胆总管受肿瘤挤压,粘连致密,锐性分离粘连,带子悬吊。游离胆管右侧显露门静脉,管壁受肿块挤压,锐性分离粘连。(LN (箭头所指):淋巴结CBD:胆总管PV:门静脉PaH:胰头)
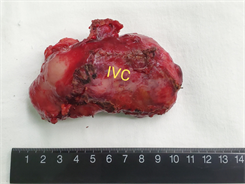
Figure 3. Adhesion of the posterior wall of the separated artery to the tumor, and free common hepatic artery, gastroduodenal artery (GDA), left hepatic artery (LHA), and right hepatic artery (RHA). Suspension. The hepatoduodenal ligament was skeletonized and the lymph nodes (arrow) were completely removed. Remove the gallbladder. (IVC: Inferior vena cava; CBD: common bile duct; CD: cystic duct; CA: cystic artery; PV: portal vein). Lymph node gross specimen: 1. Hilar giant lymph node about 10 × 6 × 5 cm, dorsal inferior vena cava IVC remember. 2. The arrow indicates frontal portal PV and hepatic artery HA
图3. 分离动脉后壁与肿瘤的粘连,游离肝总动脉、胃十二指肠动脉(GDA)、肝左动脉(LHA)、肝右动脉(RHA)。悬吊。骨骼化肝十二指肠韧带,完整切除淋巴结(箭头所指为左尾状叶)。切除胆囊。(IVC:下腔静脉;CBD:胆总管;CD:胆囊管;CA:胆囊动脉;PV:门静脉)。淋巴结大体标本:1. 肝门巨大淋巴结约10 × 6 × 5 cm,背侧下腔静脉IVC切记。2. 箭头所指为正面门静脉PV切记及肝动脉HA切记。
图4. 游离门脉右前支(RAP),给予阻断,见肝脏右前叶缺血线充分游离肝周韧带,距肿瘤边缘约3 cm设置切除线,完整切除肿瘤。肝内肿瘤大小5 × 4 × 3 cm
术后病理提示:肝右叶中–低分化胆管细胞癌,肿瘤大小约5 × 3.5 × 3 cm,切缘未见癌(图5),伴(8、12、13组淋巴结)转移癌并融合肿块,大小约9.5 × 5 × 4.5 cm (图6)。免疫组化结果:Heptocyte (−),GPC-3 (−),Arg-1 (灶状+),CK7 (−),Ki67 (+50~75%),CK19 (−)。
患者术后8天拔除全部腹腔引流管出院,围手术期恢复顺利,无任何手术并发症。术后随访:2020-11-14我院复查MRI:未见明确新生病灶,化验无异常指标。随访至今患者一般情况良好,未发现转移复发。
3. 讨论
肝内胆管细胞癌(Intrahepatic Cholangio Carcinoma, ICC)是指发生在包括二级胆管在内的末梢侧胆管上皮细胞恶性肿瘤,其发病率继肝细胞癌之后,居原发性肝脏肿瘤的第2位,占其中的10%~15% [1] [2]。随着诊疗手段的进步以及科学研究的不断深入,临床中对于ICC患者治疗策略层出不穷,但是不幸的是仍有2/3的患者在五年内复发 [3],其五年生存率波动在15%~45% [4]。
目前外科干预仍然是ICC患者惟一可能治愈的手段,但在肝内胆管癌的研究中,是否行淋巴结清扫尚存在争议。Omar Hyder等人通过汇集了从1990年5月至2011年12月多中心514病例研究美国、欧洲、亚洲等13个肝胆胰中心的表明肝内胆管细胞癌的是否发生淋巴结转移为影响预后的危险因素之一 [5]。同样我国学者沈峰对1370例肝内胆管细胞癌肝切除术后的患者分析提示:CA 19-9 > 39 U/mL、CEA > 10 μg/mL、肿瘤直径 > 5 cm、肿瘤多发、血管侵犯、淋巴结转移(HR = 1.641~2.314, P < 0.05)和局部侵犯是影响ICC肝切除术后预后的独立危险因素 [6]。
但是一篇汇集2000年1月至2018年1月共计1377例行肝内胆管细胞患者是否行淋巴结清扫的meta-分析研究表明 [7]:是否行淋巴结清扫,并不影响患者的总体生存率、无病生存率、以及复发率(总生存率 (HR 1.13, 95% CI 0.94~1.36; P = 0.20)、无病生存率(HR 1.23, 95% CI 0.94~1.60; P = 0.13),或复发率(OR 1.39, 95% CI 0.90~2.15; P = 0.14)),但是淋巴结清扫却显著增加了患者的术后并发症率(OR 2.67, 95% CI 1.74~4.10; P < 0.001)。
针对于上述研究差异,我们团队认为可能与不同肝胆外科手术医生的淋巴结清扫的程度以及要求不同相关,致使在Meta分析中存在较大的异质性。所以目前AJCC指南建议肝内胆管细胞癌的淋巴结清扫获取淋巴结应至少大于6个 [8]。针对可切除的肝内胆管癌,我们团队常规清扫肝十二指肠韧带、肝固有、胰头上方、后方、以及胃左淋巴结。同时有研究标准化淋巴结清扫范围后,发现淋巴结清扫可以显著延长无病生存率 (无清扫vs淋巴结清扫:20.0 [4.2~35.8]个月vs 64.0 [27.3~120.8]个月,P = 0.077)以及淋巴结清扫组的总生存率明显延长(未清扫组vs.淋巴结清扫组:44.0 [31.1~56.9]个月vs. 90.0 [51.1~158.9]个月,P = 0.027) [9]。但是上述研究患者入组例数较少,淋巴结清扫组及对照组仅各34例,缺乏多中心大样本量研究。
在行淋巴结清扫手术时,因肝门部解剖结构多而复杂,且常有变异,致使存在意外损伤胆管、血管风险。同时当肿瘤或转移淋巴结压迫或侵犯门静脉,肝动脉时,尤其是当需要门静脉重建,肝动脉外鞘剥离甚至重建时,手术难度较大,致使术后出现并发症率风险增高。本病例特点为肝门部(8、12、13组淋巴结)转移癌并融合肿块,大小约9.5 × 5 × 4.5 cm,位于肝十二指肠韧带内管道结构间隙之间,重要结构受癌肿挤压粘连致密,手术操作受限,难度更是极高。但是对于受过专业训练的肝胆外科医师来说风险是相对可控的。这需要手术医师术前具备精准的阅片技术,可以对肿瘤及肝内外管道结构术前进行重建,使肿瘤位置,血管胆管走形了然于胸,可以提高手术的安全性 [10]。其次术中对肝门解剖结构的熟悉、精细的操作、血管吻合的技能也是手术成功必不可少的因素之一。
4. 结论
综上所述淋巴结清扫对于可切除的肝内胆管细胞癌的患者可能是受益的,但是仍需要临床试验以及肝内胆管细胞癌区域淋巴结转移途径的基础研究进一步证实其必要性。
申明
该病例报道获得病人及家人的知情同意!
NOTES
*通讯作者。