摘要: 目的:探讨恶性腹膜间皮瘤(malignant peritoneal mesothelioma, MPM)的临床特点、病理特征、治疗及预后。方法:选取2010年至2020年在青岛大学附属医院经病理确诊的26例腹膜恶性间皮瘤患者,收集其性别、年龄、临床表现、辅助检查、病理结果、治疗方式及预后等临床资料,回顾性分析并总结其临床、病理特点及疗效。结果:26例患者中位年龄59.5岁,男女比例1:1.89,仅1例具有石棉接触史。首发症状主要表现为腹痛、腹胀,肿瘤标记物以CA125升高为主,B超和CT检查可见腹水、腹膜增厚和腹盆腔肿块,大网膜呈“饼状”。26例均经病理确诊,组织学分型:上皮型9例,肉瘤型2例,未分型15例。免疫组化指标选择不完全相同,阳性率最高的依次为CK (100%)、CK7 (94.4%)、Vimentin (90.9%)、Calretinin (89.5%)、D2-40 (88.9%)、CK5/6 (88.2%)和WT-1 (81.3%),检测CK20、CDX-2均为阴性。13例接受剖腹手术或腹腔镜手术联合全身化疗,8例接受单纯化疗,3例放弃治疗,失访2例。全部患者的中位无进展生存期(progression-free survival, PFS) 6个月,中位总生存期(overall survival, OS) 7个月。单因素生存分析显示,仅年龄的差异有统计学意义(P = 0.001, χ2 log-rank = 10.424)。结论:恶性腹膜间皮瘤临床表现无特异性,超声或CT引导下组织活检有助于诊断。恶性腹膜间皮瘤预后差,年龄影响其预后,手术和化疗不能有效延长患者生存期。
Abstract:
Objective: To discuss the clinical characteristics, pathological characteristics, treatment and prognosis of malignant peritoneal mesothelioma. Methods: Select 26 patients with peritoneal malignant mesothelioma diagnosed pathologically in The Affiliated Hospital of Qingdao University from 2010 to 2020. Collect their gender, age, clinical manifestations, auxiliary examinations, pathological results, treatment methods and prognosis and other clinical data for retrospective analysis and summarize its clinical, pathological characteristics and efficacy. Results: The median age of the 26 patients was 59.5 years, the male to female ratio was 1:1.89, and only 1 patient had a history of asbestos exposure. Initial symptoms are mainly abdominal pain, bloating. The tumor markers were mainly elevated CA125. B-ultrasound and CT examination showed ascites, thickening of the peritoneum, abdominal and pelvic masses, and a “cake-like” appearance of the omentum. All 26 cases were diagnosed by pathology, histological classification: 9 cases of epithelial type, 2 cases of sarcoma type, and 15 cases of unclassified type. The selection of immunohistochemical indexes is not exactly the same. The highest positive rate is CK (100%), CK7 (94.4%), Vimentin (90.9%), Calretinin (89.5%), D2-40 (88.9%), CK5/6 (88.2%) and WT-1 (81.3%), the detection of CK20 and CDX-2 were all negative. 13 cases received laparotomy or laparoscopic surgery combined with systemic chemotherapy, 8 received chemotherapy alone, 3 gave up treatment, and 2 were lost to follow-up. The median progression-free survival (PFS) of all patients was 6 months, and the median overall survival (OS) was 7 months. Univariate survival analysis showed that only the difference in age was statistically significant (P = 0.001, χ2 log-rank = 10.424). Conclusion: The clinical manifestations of malignant peritoneal mesothelioma are not specific. Ultrasound or CT-guided tissue biopsy is helpful for diagnosis. Malignant peritoneal mesothelioma has a poor prognosis, and age affects its prognosis. Surgery and chemotherapy cannot effectively prolong the survival of patients.
1. 引言
恶性腹膜间皮瘤(malignant peritoneal mesothelioma, MPM)是一种起源于腹膜间皮细胞的高侵袭性肿瘤,临床少见。该病起病隐匿,临床表现缺乏特异性,诊断较为困难,且预后较差。目前主要治疗方法有减瘤手术(cytoreductive surgery, CRS)、腹腔热灌注化疗(heated intraperitoneal chemotherapy, HIPEC)、全身化疗等。本文通过回顾性分析26例MPM患者的临床资料,进一步探讨其临床特点及预后因素。
2. 对象与方法
1) 对象选取2010年至2020年青岛大学附属医院经病理确诊的恶性腹膜间皮瘤患者26例,病理学诊断符合2012年美国《间皮瘤病理学诊断指南》 [1]。
2) 方法收集26例恶性腹膜间皮瘤患者的一般情况、临床表现、病理特点、治疗及转归等临床资料,对其进行回顾性总结,并分析疗效及预后因素。
3) 统计学处理采用SPSS 26.0统计软件进行统计分析。应用Kaplan-Meier法绘制生存曲线,生存曲线的比较采用Log-rank 检验。P < 0.05为差异有统计学意义。
3. 结果
1) 一般资料26例MPM患者年龄25~78岁,中位年龄59.5岁;男9例,女17例,男女比例1:1.89;1例患者既往结核病史5年,1例患者石棉接触史20余年;9例(34.6%)患者来自城市地区,17例(65.48%)患者来自农村地区。
2) 临床表现首发症状主要表现为腹胀l5例(57.8%),其次为腹痛5例(19.2%),自扪及腹部肿块2例(7.7%),不规则阴道流血2例(7.7%),发热1例(3.8%),产检发现1例(3.8%)。伴腹水、胸水18例(69.2%),伴消瘦9例(34.6%),伴发热、乏力5例(19.2%)。
3) 实验室检查26例患者中,25例行血清肿瘤标记物检测,糖类抗原125(CA125)升高12例(48%),糖类抗原199 (CA199)升高1例(4%),糖类抗原153 (CA153)升高5例(20%),癌胚抗原(CEA)升高2例(8%),甲胎蛋白(AFP)升高2例(8%)。送检腹水12例、胸水2例,腹水发现恶性肿瘤细胞6例(阳性率50%),2例胸水中均查找到肿瘤细胞。
4) 影像学检查行超声检查的15例MPM患者中,发现腹盆腔异常回声者9例(60%,排除子宫肌瘤引起的低回声),超声发现腹水12例(80%)、腹膜网膜增厚5例(33%)。25例患者接受CT检查,19例(76%)患者检测到盆腹腔积液,18例(72%)患者腹膜网膜结节样或饼样增厚,17例(68%)发现异常密度灶,淋巴结肿大5例(20%)。
5) 诊断术前疑诊恶性腹膜间皮瘤2例(7.7%),误诊为卵巢癌9例(34.6%),结核性腹膜炎4例(15.4%),胃肠肿瘤6例(23.1%),腹膜后肿物2例(7.7%),肝占位、肝硬化、前列腺癌各1例(3.8%)。16例(61.5%)经超声或CT引导下穿刺活检确诊,8例(30.8%)经术后大病理确诊,2例(7.7%)经腹水细胞学确诊。26例MPM患者的主要特征见表1。

Table 1. Main characteristics of the 26 MPM patients
表1. 26例MPM患者的主要特征
6) 组织病理及免疫组化26例MPM患者均经病理检查确诊。组织学分型:上皮型9例,肉瘤型2例,未分型15例。免疫组化指标阳性率最高的依次为细胞角蛋白(Cytokeratin, CK)、CK7、波形蛋白(Vimentin)、钙视网膜蛋白(Calretinin)、淋巴管内皮细胞特异性标志物(D2-40)、CK5/6、肾母细胞瘤蛋白1 (Wilms tumor1, WT-1),具体百分比见表2。检测细胞角蛋白20 (CK20) 12例、尾型同源盒转录因子-2 (CDX-2) 13例,均为阴性。
7) 治疗及预后26例患者有13例接受剖腹探查或腹腔镜手术及全身化疗,8例接受全身化疗,3例放弃治疗,失访2例。接受手术治疗的13例患者:11例行肿瘤细胞减灭术,2例仅行腹腔镜活检术;3例患者因术前误诊卵巢癌行紫杉醇联合铂类先期化疗1~2周期,术后接受1周期紫杉醇 + 卡铂化疗后更换化疗方案培美曲塞 + 顺铂,其余10例均接受培美曲塞 + 顺铂一线化疗,化疗1~9个周期不等。8例接受全身化疗:3例行培美曲赛 + 顺铂腹腔灌注联合静脉化疗,3例仅行培美曲塞 + 顺铂静脉化疗,1例行紫杉醇 + 奈达铂静脉化疗,化疗1~6个周期不等。1例因不能耐受全身化疗选用口服阿帕替尼治疗。

Table 2. Main immunohistochemical indexes and expression of the 26 MPM patients
表2. 26例MPM患者的主要免疫组化指标及表达情况
注:CK:细胞角蛋白;Vimentin:波形蛋白;Calretinin:钙视网膜蛋白;D2-40:淋巴管内皮细胞特异性标志物;WT-1:肾母细胞瘤蛋白1;CDX-2:尾型同源盒转录因子2。
从患者确诊日期开始随访,随访至患者死亡或2021年5月,随访时间2~36个月。至随访结束,有21例患者死亡,3例存活,2例失访。随访的24例患者中,完全缓解(CR) 3例,部分缓解( partial response, PR) 6例,稳定( stable disease, SD) 8例,进展(PD) 7例。客观缓解率(overall response rate, ORR) 37.5%,疾病控制率(disease control rate, DCR) 70.8%。中位无进展生存期( progression-free survival, PFS) 6个月,中位总生存期(overall survival, OS) 7个月。生存曲线见图1。
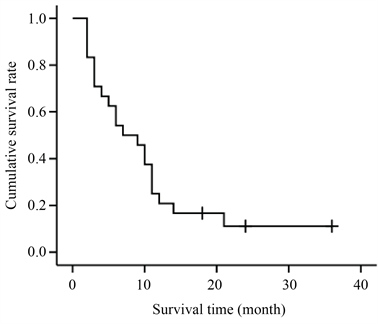
Figure 1. Kaplan-Meier survival curve of 24 MPM patients
图1. 24例MPM患者的 Kaplan-Meier生存曲线
将性别、年龄、常住地、血清CA125水平、不同治疗模式进行单因素生存分析,结果显示仅年龄的差异(P = 0.001, χ2 log-rank = 10.424)有统计学意义(表3)。生存曲线见图2。因单因素分析显示仅年龄差异有统计学意义,遂未行多因素分析。

Table 3. Univariate analysis of the prognosis in 24 MPM patients
表3. 24例MPM患者预后的单因素分析
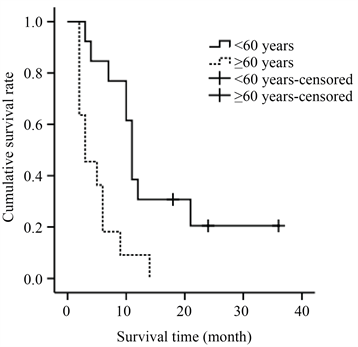
Figure 2. Kaplan-Meier survival curves of different ages
图2. 不同年龄的Kaplan-Meier生存曲线
4. 讨论
恶性腹膜间皮瘤(malignant peritoneal mesothelioma, MPM)起源于腹膜浆膜的间皮和间皮下层细胞,具有向纤维母细胞和上皮细胞双向分化的能力,恶性程度高,预后差。MPM发病率约为1/100万~2/100万,约占所有腹膜间皮瘤的20%,是临床罕见肿瘤 [2]。MPM发病高峰在55至60岁之间,但也可发生在年轻人和老年人中 [3]。本组研究患者发病年龄25~78岁,中位年龄59.5岁,发病年龄跨度较大,年轻患者的增加可能与患者自身健康意识增强、就医及时有关。石棉是已知的胸膜恶性间皮瘤的致病因素,但并非所有间皮瘤都与石棉有关,尤其女性患者可归因于石棉暴露的更少 [4]。毛沸石、碳纳米管等其他矿物质、辐射、慢性炎症、猿病毒40 (SV40)是近来研究较多的MPM病因。本研究中仅有1例男性患者有石棉接触史,其余25例均无石棉接触史,因此,其确切病因及发病机制尚有待进一步探索。
MPM起病隐匿,临床表现为腹痛、腹胀等,少数病例无症状偶然发现,确诊时多为晚期 [5]。本研究患者临床表现大体与文献报道相符,主要以腹痛、腹胀为主诉,部分女性病人以扪及腹部包块、阴道流血来诊,22例患者(84.6%)确诊时已进展至III~IV期。此外,本研究中6例患者起病时有发热,发热程度不等,且检查发现69.2%伴有腹水和或胸水。MPM临床表现的无特异性,致使该病极易被误诊为结核性腹膜炎、卵巢癌、腹膜转移癌等 [6]。本研究初诊腹膜恶性间皮瘤者仅2例(占7.7%),误诊为卵巢癌、胃肠肿瘤、结核性腹膜炎者分别占34.6%、15.4%、23.1%,误诊率较高。血清肿瘤标志物以CA125升高为主,本组占40%,部分患者有CA153、CEA和AFP升高,但无特异性。超声、CT作为临床常用辅助检查,对早期发现腹水、腹腔内实质性肿块具有较大优势。该病超声表现多可见腹膜、大网膜弥漫性增厚,厚薄不均,探头触之质硬。CT表现的直接征象包括大网膜、肠系膜弥漫性增厚及不规则和(或)结节状增厚 [7]。二者可及时发现大网膜病变及病变位置与范围,并为腹膜穿刺定位提供一定的依据与帮助。本研究61.5%患者行超声或CT引导下穿刺活检,在尽可能小的创伤前提下得到了病理学诊断。
PMM组织病理学上具有双向分化特点,分为3种类型即上皮型、肉瘤型和混合型,但存在多种变体,病理组织结构复杂 [8]。BRCA相关蛋白1 (BAP1)和MTAP免疫组化和CDKN2A荧光原位杂交可辅助分离良性和恶性间皮细胞增生 [9]。Tandon等人通过对244例PMM分析发现,敏感的免疫组化标记物包括Calretinin (100%)、WT-1 (94%)、CK5/6 (89%)和D2-40 (80%),BAP1在PMM中失表达具有高度特异性 [10]。由于本研究为回顾性分析,本研究组患者的免疫组织化学染色指标的选择不完全相同,这是因为目前仍未有任何一种抗体对MPM完全特异和敏感 [11]。本研究采用的免疫组化指标中,阳性率最高的依次为CK (100%)、CK7 (94.4%)、Vimentin (90.9%)、Calretinin (89.5%)、D2-40 (88.9%)、CK5/6 (88.2%)和WT-1 (81.3%),检测CK20、CDX-2均为阴性。本研究与Tandon等人得出的结论不完全相同,可能是由于本研究样本量较小,同时也说明需进一步探索可诊断MPM的特异性免疫组化指标。
MPM缺乏规范有效的治疗方法,肿瘤细胞减灭术(cytoreductive surgery, CRS)联合腹腔热灌注化疗(hyperthermic intraperitoneal chemotherapy, HIPEC),目前被认为是治疗PMM的首选方法;铂类联合培美曲塞是PMM首选化疗药物。一项纳入了1047例肿瘤细胞减灭术联合腹腔热灌注化疗的MPM患者的Meta分析显示,患者的1、3、5年生存率分别为84%、59%和42% [12]。Salo SAS等人报道CRS加HIPEC和根治性手术联合化疗可提高患者生存率 [13]。此外,HIPEC联合常温腹腔内长期化疗(normothermic intraperitoneal chemotherapy long-term, NIPEC-LT)亦在MPM患者中得到有效利用 [14]。在没有CRS和HIPEC的情况下,MPM患者的中位生存期约为1年。积极的手术方法加上局部化疗使中位生存期增加到5年以上 [15]。CRS、HIPEC和NIPEC-LT的使用使MPM患者受益颇多。本研究11例患者进行减瘤手术,3例患者进行腹腔热灌注化疗,统计结果显示,接受减瘤术的患者OS未见明显延长,这可能是由于患者病情已进展至晚期及患者全身状况较差,且本组患者多来自农村地区,经济条件限制使其未能接受完整的巩固化疗。本研究大部分患者选择培美曲塞为基础的全身化疗,分析结果显示,培美曲塞化疗组较其他药物组无生存优势,可能与样本量小有关。本研究应用的紫杉醇联合铂类化疗的相关研究数据较少,尚需进一步扩大样本量以探究其意义。法国试验组在欧洲肿瘤内科学会会议上报告的一项试验评估了将贝伐单抗添加到培美曲塞和铂治疗胸膜间皮瘤的效用。该研究发现,加入贝伐单抗后,患者中位OS时间从2.7个月增加到近19个月。因此,这种三药方案逐渐成为许多中心当前MPM的治疗选择 [16]。
MPM临床少见,预后差,不同学者对不同地区MPM预后影响因素分析不尽相同 [17] [18] [19] [20]。认可较多的独立预后因素包括年龄、性别、组织学亚型、细胞减灭术的完整性、腹膜癌指数(peritoneal cancer index, PCI)和淋巴结转移。Chapel DB等研究显示,年龄 < 60岁、ECOG体能状态0或1、影像学检查无淋巴结转移、细胞减灭术、腹腔热灌注化疗、腹膜癌指数 < 27和上皮样组织亚型为有利的独立预后因素 [17]。Yonemura Y等认为HIPEC、腹膜癌指数(PCI)评分C12、无远处转移和组织学上皮类型是MPM患者预后的独立影响因素 [18]。Magge D等人研究显示,年龄及性别是预后的独立影响因素 [19]。而Yin等人研究报道高血液中性粒细胞与淋巴细胞比率(neutrophil-to-lymphocyte ratio, NLR)和低蛋白血症是MPM患者的不良预后因素 [20]。本研究结合临床常用的指标,评估了MPM患者的预后影响因素,仅年龄的差异具有统计学意义,但本组患者例数相对较少,还需大样本进一步研究。
5. 总结
综上,本研究回顾性分析了26例腹膜间皮瘤患者的临床及病理特征、治疗及转归,由于MPM为罕见病,样本量小,为回顾性分析,部分统计学分析未得到差异有统计学意义的结论,仍需扩大样本量进一步研究。期待有更多关于治疗模式、靶向药物、生物标记物方面的前瞻性随机对照研究,以期延长腹膜间皮瘤患者的生存。
NOTES
*通讯作者Email: chenaiping516@163.com