摘要: 目的:分析受控衰减参数(controlled attenuation parameter, CAP)以及FAST评分在评估肝移植(Liver transplantation, LT)后新发非酒精性脂肪肝(nonalcoholic fatty liver disease, NAFLD)中的应用价值。方法:这是一项单中心、前瞻性研究,对2014~2021年接受LT并在青岛大学附属医院器官移植中心规律随访的成年LT受者进行腹部超声检查及瞬态弹性成像(Transient elastography, TE)检测,建立受试者工作(receiver operating characteristic, ROC)曲线分析CAP在评估LT后新发NAFLD方面的应用价值,并通过计算FibroScan-天冬氨酸氨基转移酶评分(FibroScan-aspartate aminotransferase score, FAST)以评估LT后新发NAFLD患者的非酒精性脂肪性肝炎(nonalcoholic steatohepatitis, NASH)进展风险。结果:本研究对符合纳入标准的113例LT受者进行了TE检测,LT手术至TE检测的中位时间为3.3 (2.2~4.5)年,新发NAFLD的发病率为35.4% (40/113),其中,52.5% (21/40)为轻度NAFLD,22.5% (9/40)为中度NAFLD,25.0% (10/40)为重度NAFLD。CAP诊断LT后新发NAFLD、LT后中–重度NAFLD及重度NAFLD的ROC曲线下面积分别为0.80 (0.70~0.90)、0.95 (0.89~1.00)和0.99 (0.97~1.00),截止值分别为240.5 dB/m、264.5 dB/m和296.0 dB/m,且FAST值与LT后NASH进展高风险密切相关(P = 0.001)。单因素分析表明,LT后新发NAFLD患者的白蛋白水平(P = 0.039)、ALT水平(P = 0.029)、AST水平(P = 0.004)以及重度NAFLD (P = 0.044)与NASH高风险显著相关,而多因素分析提示,AST水平(OR 1.394, 95%CI 1.052~1.847, P = 0.021)是LT后新发NAFLD患者NASH进展高风险的独立危险因素。结论:CAP是LT后新发NAFLD患者诊断和严重程度分级的良好工具,具有良好的敏感性和特异性,尤其是在中度至重度LT后新发NAFLD中诊断性能尤为突出,可以用于量化LT后新发NAFLD。在LT后新发NAFLD患者中,FAST评分可用来评估其NASH进展风险,监测AST水平对预防LT后新发NAFLD患者NASH进展具有重要意义。
Abstract:
Aims: This study aimed to determine of the utility of controlled attenuation parameter (CAP) and FibroScan-AST (FAST) score in the evaluation of de-novo nonalcoholic fatty liver disease (de-novo NAFLD) after liver transplantation (LT). Methods: In this prospective, single-center study, ab-dominal ultrasound and Transient Elastography (TE) were performed for adult patients who un-derwent LT between 2014 and 2021, and were regularly followed up at the Organ Transplantation Center of the Affiliated Hospital of Qingdao University. Receiver operating characteristics (ROC) curve was constructed to determine the diagnostic performance of CAP in the detection of de-novo NAFLD after LT, and the FAST score was calculated to assess de-novo NAFLD patients at risk of pro-gressive NASH. Results: A total of 113 LT recipients were included in the study. The median period from LT to TE detection was 3.3 (2.2~4.5) years. The incidence of de-novo NAFLD was 35.4% (40/113), of which 52.5% (21/40) had mild NAFLD, 22.5% (9/40) had moderate NAFLD, and 25.0% (10/40) had severe NAFLD. CAP diagnosed de-novo NAFLD, moderate-severe NAFLD, and severe NAFLD with the area under the ROC curves (AUROC) mean 0.80 (0.70~0.90), 0.95 (0.89~1.00), and 0.99 (0.97~1.00), respectively. The cut-off values were 240.5 dB/m, 264.5 dB/m, and 296.0 dB/m, respectively. The FAST score identifying the risk of progressive NASH was significantly correlated with the severity of de novo NAFLD after LT (P = 0.001). Univariate analysis demonstrated that al-bumin levels (P = 0.039), ALT levels (P = 0.029), AST levels (P = 0.004), and severe NAFLD (P = 0.044) were significantly associated with high risk of NASH in patients with de-novo NAFLD after LT. The multivariate analysis suggested that AST (OR 1.394, 95%CI 1.052 to 1.847; P = 0.021) was an independent risk factor for NASH progression in de-novo NAFLD patients after LT. Conclusions: CAP provides an efficient way for the diagnosis and severity classification of patients with de-novo NAFLD after LT, especially in those with moderate to severe NAFLD, which can be applied to quantify de-novo NAFLD after LT. In patients with de-novo NAFLD after LT, FAST score can be used to assess the risk of NASH progression, and serum AST level is an important index for predicting NASH pro-gression.
1. 前言
肝移植(Liver transplantation, LT)是治疗各种终末期肝病最有效的治疗方法 [1] 。随着肝移植技术的发展,围术期管理水平的提高以及术后免疫抑制剂的应用,肝移植的长期结果得到改善,肝移植后的10年生存率可达65% [2] 。随着肝移植术后存活时间的延长,移植后代谢综合征及其相关并发症的发病率逐渐升高,尤其是非酒精性脂肪肝病(nonalcoholic fatty liver disease, NAFLD)和非酒精性脂肪性肝炎(nonalcoholic steatohepatitis, NASH),已经成为LT后常见的慢性并发症 [3] 。LT后新发NAFLD可进展为肝纤维化,而且与许多肝外慢性疾病有关,是心血管动脉粥样硬化疾病和LT术后新发肝外实体恶性肿瘤(如结肠癌、胰腺癌)的重要风险因素,与LT后无NAFLD的受者相比,LT后新发NAFLD受者发生心血管动脉粥样硬化疾病和术后新发肝外恶心肿瘤的相对风险比(Hazard ratio, HR)可达1.048和2.214;而新发NASH则是LT后远期死亡的独立危险因素,HR可达4.133 [4] [5] 。因此,早期发现和评估LT后新发NAFLD及NASH是预防其不良后果的关键。
肝活检是评估肝移植术后肝脂肪变性和肝纤维化的金标准,然而,肝活检是一项侵入性操作,有出血、疼痛、感染等并发症风险,临床推广难度较大 [6] 。腹部超声是临床常用的NAFLD的筛查方法,然而,超声检查无法实现对肝脏脂肪变性的定量评估,也无法精确评价肝纤维化情况 [7] 。瞬态弹性成像(Transient elastography, TE)可通过受控衰减参数(controlled attenuation parameter, CAP)和肝硬度测量(Liver stiffness measurements, LSM)同时准确评估肝脏脂肪变性和肝纤维化 [8] [9] [10] 。国外研究报道,与肝活检相比,CAP可准确检测LT受者的肝脂肪变性,根据受试者工作曲线(receiver operating characteristic, ROC)分析得出,CAP检测LT后肝脏脂肪变性的受试者工作曲线下面积(area under the receiver operating characteristic, AUROC)为0.88,检测LT后中重度肝脏脂肪变性的AUROC可达0.94,且具有无创的优越性 [11] 。FibroScan-天冬氨酸氨基转移酶评分(FibroScan-aspartate aminotransferase score, FAST)是一种可用于识别NAFLD患者中具有进展性NASH和肝纤维化高风险人群的新型预测模型,且与肝活检相比,FAST评分的诊断性能良好 [12] [13] ,但其在LT受者新发NAFLD中的应用价值尚未见报道。
本研究旨在描述LT后新发NAFLD患者的发病率及临床特点,并评估CAP在诊断及评估LT受者新发NAFLD方面的表现特征,同时通过FAST评分初步评估LT后新发NAFLD患者的NASH进展风险。
2. 方法
2.1. 研究设计
这是一项单中心、前瞻性研究。于2021年3月至2022年4月对在青岛大学附属医院器官移植中心规律随访的成年LT受者进行腹部超声检查及TE检测,以评估LT受者肝脏脂肪变性及肝纤维化情况。本研究符合《赫尔辛基宣言》的道德准则,并经过青岛大学附属医院伦理委员会审批。入选标准:1) 2014至2021接受肝移植手术并在青岛大学附属医院规律随访的成年患者;2) 自愿接受腹部超声检查及TE检测;3) 移植物功能基本正常。排除标准为:1) 年龄 < 18岁;2) 血清谷丙转氨酶升高超过正常上限5倍;3) 血清总胆红素升高超过正常上限3倍;4) LT后过量饮酒患者(男性每天饮酒>20克,女性每天饮酒>10克);5) 伴右心衰竭的患者。
2.2. TE检测
所有患者在禁食12小时后接受TE检测,由有经验的操作员执行检测,受试者为仰卧位,右手放在头后,右臂最大外展,暴露肝右叶区的肋间隙,在同一检测点至少检测12次,检测值四分位数差距与中位数比值 < 0.3,认为检测可靠 [14] 。
2.3. 超声检查
所有患者均在TE检测前后1周内接受了腹部超声检查,评估是否有肝脏脂肪变性,并对脂肪变性程度做出评估(轻度、中度、重度) [7] 。
2.4. 数据收集
入组后,研究者调查了所有受试者的高血压、糖尿病及代谢综合征等慢性病史、肝移植手术病史及近1月服用免疫抑制剂情况等,并测量患者的身高、体重、腰围、血压。患者接受TE检测后,记录所测量的CAP及LSM值,分别用dB/m和kPa表示。所有入组患者接受实验室检测,包括血小板计数、丙氨酸氨基转移酶(ALT)、天冬氨酸氨基转移酶(AST)、碱性磷酸酶(AP)、γ-谷氨酰转移酶(GGT)、胆红素、白蛋白、肌酐、尿酸、葡萄糖、总胆固醇、甘油三酯、低密度脂蛋白胆固醇(LDL)及高密度脂蛋白胆固醇(HDL)水平,并计算FAST评分,具体计算方式为:FAST = (e − 1.65 + 1.07 × ln (LSM) + 2.66 × 10 – 8 × CAP3 − 63.3 × AST-1)/(1+ e − 1.65 + 1.07 × ln (LSM) + 2.66 × 10 − 8 × CAP3 − 63.3 × AST-1) [12] 。
2.5. 相关定义
根据2020年发布的《JSGE/JSH循证临床实践指南:非酒精性脂肪性肝病/非酒精性脂肪性肝炎的管理》,NAFLD的定义为通过影像学(超声)检查发现肝脏脂肪变性,并排除其他肝病,如酒精性肝病、病毒性肝病和药物性肝病 [15] 。
LT术后新发NAFLD,指因NAFLD以外的肝脏疾病接受LT的受者术后首次经影像学诊断为NAFLD [16] [17] 。
根据之前的研究结果,NAFLD患者中FAST评分 ≥ 0.35则定义为具有NASH进展风险 [12] 。
高血压定义为收缩压 ≥ 140 mmHg和(或)舒张压 ≥ 90 mmHg,或平素口服降压药物控制血压 [18] 。糖尿病定义为空腹血糖 ≥ 7.0 mmol/L或平素口服降糖药物或使用胰岛素控制血糖 [19] 。
依据2018年发布的《非酒精性脂肪性肝病的预防和治疗指南》,代谢综合征的诊断应满足以下三个及以上的代谢风险因素:1) 腹部肥胖(男性腰围 > 90厘米,女性腰围 > 85厘米);2) 动脉高血压(动脉血压 ≥ 130/85 mmHg或接受降压治疗);3) 高甘油三酯血症(空腹血清甘油三酯 ≥ 1.7 mmol/L或服用降脂药物);4) 低-高密度脂蛋白胆固醇(男性HDL-C < 1.0 mmol/L,女性HDL-C < 1.3 mmol/L);5) 高血糖(空腹血糖 ≥ 5.6 mmol/L或餐后2小时血糖 ≥ 7.8 mmol\/L,或有2型糖尿病病史) [20] 。
2.6. 统计分析
收集的临床数据使用IBM SPSS Statistic for Windows (22.0版,IBM公司,美国纽约)进行统计分析。连续变量用均数 ± 标准差或中位数(四分位范围)表示,分类变量用百分比表示,连续变量的缺失值用均值填补法填补,分类变量的缺失值使用虚拟变量法填补。t检验或非参数检验用于比较连续变量与结果的相关性,Fisher精确检验或卡方检验则用于分类变量,logistic回归用于影响因素的分析。构建受试者工作曲线(receiver operating characteristic, ROC),使用曲线下面积(AUROC)和95%置信区间(CI)分析CAP在诊断LT后新发NAFLD及评估其不同严重程度的敏感性、特异性、阳性预测值(PPV)、阴性预测值(NPV)。
3. 结果
3.1. 研究人群
2021年3月至2022年4月,共有439名LT受者于青岛大学附属医院器官移植中心接受随访,其中49.7% (218/439)的患者在本诊疗组接受随访观察,其中,11名患者在此期间因LT术后相关并发症去世,40名患者转氨酶升高超过正常上限5倍,20名患者在随访期间出现明显黄疸,18名患者腹部超声数据缺失,16名患者拒绝接受TE检测,最终,共有113例LT受者(105例于本院接受LT手术,8例于外院接受LT手术,但术后于本院规律随访)符合纳入标准并入组。
3.2. 一般特征(见表1)

Table 1. Clinical and biochemical of characteristics of LT recipients
表1. LT受者的临床和生化特征
113名LT受者中,78.8%为男性(89/113),肝移植时的平均年龄为50.2 (25~70)岁。最常见的LT适应症是肝细胞癌(49.6%, 56/113),其次是良性乙肝相关性终末期肝病(21.2%, 24/113)。105名本院肝移植受者中33.3% (35/105)有供肝脂肪变性,8名外院接受肝移植的患者供肝病理缺失。113名LT受者从LT到接受TE检测的中位时间为3.3 (2.2~4.5)年,接受检测时的平均年龄为53.6 (28~74)岁,CAP中值为225 (181~255.5) dB/m,LSM的中值为6.0 (4.8~8.3) kPa。根据腹部超声结果,LT后新发NAFLD的发病率为35.4% (40/113),其中,52.5% (21/40)为轻度NAFLD,22.5% (9/40)为中度NAFLD,25.0% (10/40)为重度NAFLD。
3.3. CAP在LT后新发NAFLD诊断和分级中的临床价值
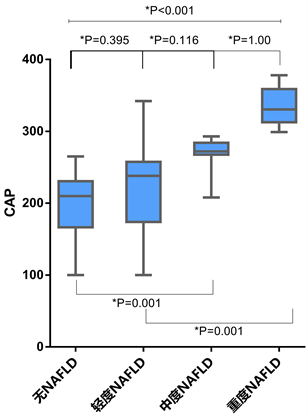
Figure 1. Grades and CAP values for post-transplant de-novo NAFLD
图1. 移植后新发非酒精性脂肪肝的等级和CAP值
LT后NAFLD组、中重度和重度NAFLD组的CAP中值分别为210.0 (166.5~230.5)、238.0 (174.0~ 257.5)、272.0 (267.5~284.0)、330.5 (312.5~358.8) dB/m,将LT后无NAFLD组、轻度、中度和重度NAFLD组分别记为0、1、2、3级脂肪变性,各级脂肪变性的CAP值总体显著增加(P < 0.001),但是,通过事后多重比较,CAP值在无NAFLD组与中度NAFLD组(P = 0.001)、无NAFLD组与重度NAFLD组(P < 0.001)、轻度NAFLD组与重度NAFLD组(P = 0.001)之间差异最为显著(见图1)。用于区分0级与1~3级脂肪变性、0~1级与2~3级脂肪变性、0~2级与3级脂肪变性的ROC曲线下面积分别为0.80 (0.70~0.90)、0.95 (0.89~1.00)和0.99 (0.97~1.00) (见表2,图2)。使用约登指数计算的用于区分0级与1~3级脂肪变性、0~1级与2~3级脂肪变性、0~2级与3级脂肪变性的临界值分别为240.5 dB/m、264.5 dB/m和296.0 dB/m (见表2)。

Table 2. Diagnostic performance of CAP assessing post-transplant de novo NAFLD Grade
表2. CAP评估移植后新发非酒精性脂肪肝等级的诊断性能
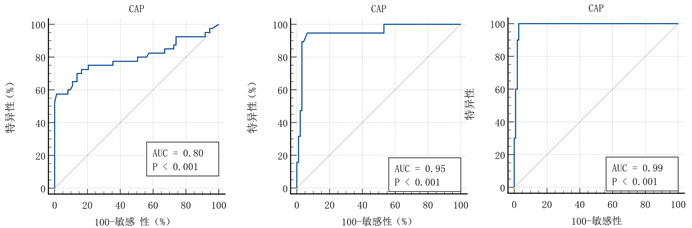 (a) (b) (c)
(a) (b) (c)
Figure 2. The predictive ability of CAP for diagnosing post-transplant de novo NAFLD (a), moderate to severe (b) and severe (c) NAFLD
图2. CAP对LT后NAFLD (图(a))、中重度(图(b))和重度(图(c)) NAFLD的诊断预测能力
与LT后无NAFLD受者相比,LT后新发NAFLD受者的平均BMI (26.5 VS 23.4 kg/m2,P < 0.001)、腰围(95.6 VS 87.3 cm, P < 0.001)、CAP (260.6 VS 198.8 dB/m, P < 0.001)、LSM (9.1VS6.5 kPa, P = 0.025)、甘油三酯(1.5 VS 1.1 mmol/L, P = 0.001)水平偏高,而HDL (1.4 VS 1.6 mmol/L, P = 0.016)水平偏低。而且,LT后新发NAFLD受者的MS发病率明显高于无NAFLD受者(42.5% VS 21.9%, P = 0.021) (见表1)。
3.4. FAST评分在分析LT后新发NAFLD患者中NASH进展风险的临床价值
在113名LT受者中,无NAFLD组、轻度、中度和重度NAFLD组的平均FAST分别为0.11 (0.002~0.67)、0.17 (0.01~0.65)、0.15 (0.03~0.56)和0.39 (0.14~0.72)。FAST值与脂肪变性程度密切相关(P = 0.001),且在无NAFLD组与重度NAFLD组(P < 0.001)、轻度NAFLD组与重度NAFLD组(P = 0.006)之间差异显著(见图3)。
通过单因素分析,在LT后新发NAFLD组中,患者的白蛋白水平(p = 0.039)、ALT水平(P = 0.029)、AST水平(p = 0.004)以及重度NAFLD (P = 0.044)与LT后新发NAFLD患者NASH高风险显著相关,多因素分析中,AST水平(OR 1.394, 95% CI 1.052~1.847; P = 0.021)是LT后新发NAFLD患者NASH高风险的独立危险因素(见表3)。
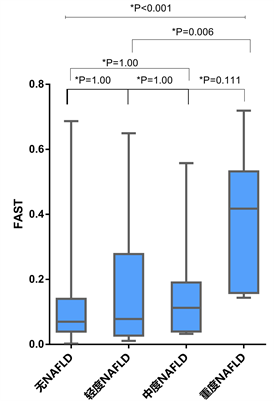
Figure 3. FAST score according to post-transplant de novo NAFLD grade
图3. LT后不同严重程度的NAFLD患者的FAST箱式图

Table 3. Logistic regression analysis of risk factors for NASH progression in NAFLD patients after liver transplantation
表3. 肝移植后NAFLD患者发生NASH的危险因素Logistic回归分析
4. 讨论
本研究是一项前瞻性研究,通过对113例LT的患者进行TE检测发现,CAP可以准确诊断LT后新发NAFLD,尤其是对中度至重度NAFLD的诊断价值尤为突出,而且,FAST评分与LT后新发NAFLD严重程度显著相关。
LT后新发NAFLD是LT后常见的并发症之一,且与LT受者长期死亡率增加相关 [4] ,因此,LT术后新发NAFLD的综合诊治,值得临床医务工作者予以重视。Dumortier等人对421名LT受者进行了随访研究,发现了LT后3.3年新发NAFLD的发病率为31.0%,新发NASH的发病率为3.8% [21] 。而Stefano等人对194名LT受者进行了为期5年的随访,通过肝穿刺组织活检,发现LT后新发NAFLD发病率为20% [4] 。Javier等人对435名LT受者进行了长达5年的随访,在肝移植后的第一年,对LT受者每3~6月进行一次腹部超声检查,在肝移植后的3~5年,则每年进行一次腹部超声检查,结果发现LT后新发NAFLD的发病率为36.11% [16] 。而在本研究中,LT后新发NAFLD的发病率为35.4%,结果与Dumortier和Javier等人的研究结果相似,但高于Stefano等人的研究,考虑与不同中心对LT后新发NAFLD采用的诊断方法有所区别有关。
CAP是NAFLD患者脂肪变性诊断和分级的无创性工具,具有良好的敏感性和特异性,其价值在LT后新发NAFLD的诊断与分级中也得到了较好的验证 [8] 。早期研究表明,与肝组织病理相比,CAP可以准确检测出 ≥ S1级(轻度脂肪变性,脂肪变性 > 10%)和 ≥ S2级(中度脂肪变性,脂肪变性 > 33%)的肝脏脂肪变性,AUROC分别为0.91和0.95 [22] 。而最近的研究表明,CAP对肝脏脂肪变性(≥ S1级)的准确性一般(AUROC = 0.74),但对中重度脂肪变性(≥ S2级)的诊断具有极好的准确性(AUROC = 0.92) [23] 。而本研究通过腹部超声对LT后新发NAFLD的严重程度进行了轻中重分级,结果显示,CAP可较为准确的诊断出LT后新发NAFLD (AUROC = 0.8),尤其对中重度NAFLD (AUROC = 0.95)和重度NAFLD (AUROC = 0.99),其诊断价值更高,而且,LT后轻度NAFLD的CAP截止值为240.5~264.5 dB/m,中度NAFLD的CAP截止值为264.5~296 dB/m,重度NAFLD的CAP截止值为> 296 dB/m,与目前通用的CAP截止值相似 [24] ,此外,与肝脏穿刺活组织检查相比,CAP属于无创性检查,可重复进行,患者依从性较高,价格更为低廉,因此,笔者认为,在LT术后新发NAFLD的综合诊治方面,CAP的临床价值更高,值得推广。
既往研究表明 [12] [13] [25] ,作为一种可用于识别NAFLD患者中具有进展性NASH和肝纤维化高风险人群的新型预测模型,FAST评分有助于减少不必要的肝活检,同时,上述研究表明NAFLD患者的脂肪肝指数 > 63.9是NASH高风险的独立危险因素。本研究发现,FAST值与脂肪变性程度密切相关(P = 0.001),这也许提示着FAST评分亦与LT后新发NAFLD患者的NASH高风险密切相关,值得进一步研究。
本研究尚存在一定的局限性,首先,纳入本研究的样本量较少,且为单中心研究;其次,本研究缺乏LT受者的肝脏组织病理结果,仅通过腹部超声和TE检测来诊断LT后新发NAFLD并评估NASH进展风险,对研究结论有一定影响;而且,本研究将FAST评分 ≥ 0.35作为截止值来评估NAFLD患者NASH进展风险,是基于普通NAFLD人群所得,而在LT后新发NAFLD患者中尚无明确结论,日后仍需进一步研究以验证其在LT受者中的应用价值。
5. 结论
CAP是LT后新发NAFLD患者诊断和严重程度分级的良好工具,尤其是在中度至重度NAFLD中的诊断性能尤为突出,同时,FAST评分有助于评估LT后新发NAFLD患者出现NASH进展的风险,对提高LT后新发NAFLD患者综合管理水平具有重要的临床意义。
NOTES
*通讯作者Email: kongxinjuan2003@163.com