摘要:
本文考察了苯乙酮和四丁基三溴化铵(TBABr3)的溴代反应中路易斯酸对反应速率的影响。在所研究的路易斯酸的范围内,ZnCl2、AlCl3和FeCl3能加速反应的进行,但是FeCl2和NiCl2抑制了溴代反应。从结果来看,FeCl3不仅能显著缩短反应时间,同时收率也有所提高。
Abstract: We investigate the effects of some Lewis acids (ZnCl2, AlCl3, FeCl3, FeCl2, and NiCl2) on the α-bro- mination rate of acetophenone with tetrabutylammonium tribromide. It is found that some Lewis acids, such as ZnCl2, AlCl3, and FeCl3, can accelerate bromination of acetophenone, but others such as FeCl2 and NiCl2 inhibited the bromination. Among the catalytic Lewis acids, FeCl3 could significantly accelerate the reaction without loss of the yield.
1. 引言
α-溴代苯乙酮类化合物是重要的有机合成中间体,被广泛地应用于医药、农药等功能化学品的合成,例如α-溴代苯乙酮是非甾体抗炎药芳基丙酸类药物的重要中间体[1] 。以苯乙酮为原料,文献报道的溴化试剂有液溴[2] 、N-溴代丁二酰亚胺(NBS)[3] 、溴化铜[4] 、四丁基三溴化铵(TBABr3)[5] 等。液溴价格较低,但选择性较差,不可避免的产生苯环溴代物,且产生废酸而污染环境。NBS价格较高,工业上难以大规模使用。溴化铜反应速度快,但价格昂贵。TBABr3在α-溴代苯乙酮合成中,具有操作简单,合成容易,反应条件温和,后处理简单的优点,但是反应时间相对较长。
路易斯酸是指一类能接受电子对的物质,从其结构上看是一类具有空轨道的物质[6] 。由于路易斯酸具有很好的催化活性,被广泛用于有机合成当中。本文考查路易斯酸对TBABr3对苯乙酮溴代反应的影响(图1)。
2. 实验部分
2.1. 主要仪器和试剂
熔点用 XRC-1 显微熔点仪测定。
二氯甲烷(DCM)和甲醇(MeOH)经过精制除水后使用,其他的反应试剂均为市售分析纯(Acros),购买后未经处理直接使用。
2.2. 合成
(1) 四丁基三溴化铵(TBABr3)的合成
在250 mL的烧杯中加入四丁基溴化铵(TBAB) (25.00 g, 78 mmol)和溴酸钾(4.34 g, 26 mmol),再加入160 mL水,室温搅拌,全部溶解后,搅拌的同时再向其中缓慢滴加40%氢溴酸(23.00 mL)。产生的黄色沉淀经抽滤、水洗、晾干后,用DCM和石油醚混合溶剂重结晶,得到桔红色晶体35.54 g,产率95.0%。M.p.70℃~72℃,与文献值相符[7] 。
(2) 不同路易斯酸存在下α-溴代苯乙酮的合成
将苯乙酮(1 eq)溶解在DCM和MeOH的混合溶剂(体积比5:2)中,室温搅拌,再加入适量路易斯酸和TBABr3 (1 eq),继续室温搅拌直至溶液颜色褪去。减压移除溶剂。剩余物以DCM溶解,经饱和的Na2S2O3溶液洗涤,水洗后,用无水Na2SO4干燥。有机层减压浓缩后,残余物以EtOH和H2O重结晶,得到无色针状晶体。M.p.49℃~50℃,与文献值相符[8] 。产率(%) = (溴代苯乙酮的摩尔数/苯乙酮的摩尔数) × 100%。
3. 结果与讨论
以苯乙酮为原料,用TBABr3对苯乙酮的甲基进行溴代,考查不同路易斯酸对反应的影响,结果如表1所示。
实验结果表明,AlCl3、ZnCl2和FeCl3能加速反应的进行,表明这些路易斯酸对该反应有催化作用。其它的路易斯酸如FeCl2、NiCl2则没有起到催化作用且抑制了反应的进行。根据文献报道的TBABr3的溴代反应机理[5] ,苯乙酮的烯醇化是反应的控速步骤。结合实验结果,我们推测,在AlCl3、ZnCl2或FeCl3存在下,苯乙酮的烯醇化更易进行,从而加快了溴代反应。
另一方面,随着路易斯酸加入量的增加,反应产率逐渐降低。这可能是由于这些路易斯酸不仅能够
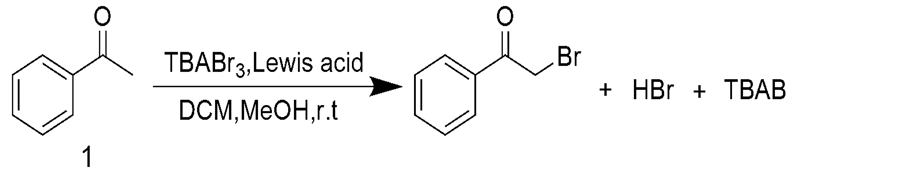
Figure 1. Bromination of acetophenone with tetrabutylammonium tribromide
图1. 四丁基三溴化铵对苯乙酮的溴代反应

Table 1. Synthesis of α-bromoacetophenone in the existence of various Lewis acids
表1. 不同路易斯酸存在下α-溴代苯乙酮的合成
加快烯醇化的进行,同时也能与TBABr3结合,加快了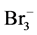 的分解,导致反应产率降低。
的分解,导致反应产率降低。