摘要: 本文利用高温氮化技术,以Ta
2O
5@Ta
3N
5为前驱体,以Co
3+为修饰剂,成功制备了Co
xN (Co
5.47N, Co
2N)表面修饰的Ta
2N/Ta
3N
5纳米叶,其可见光催化解水产氢活性高达59.2 umolg
−1∙h
−1,远高于Ta
2O
5@Ta
3N
5前驱体的21.75 umolg
−1∙h
−1。控制氮化时间调控多重异质结构的组成、电子结构、载流子界面分离和转移。构建Co
xN/Ta
2N/Ta
3N
5多重异质结显著促进了电荷载流子的分离和迁移;缺氮化物Co
5.47N产生等离子共振效应、低价Ta
2N中离域的Ta 5d及N
3-缺陷引入的杂质能级共同作用增强样品的可见光吸收,进而提升样品的光催化水解析氢性能。构建多重异质结为设计开发高效稳定的用于太阳能解水制氢的Ta
3N
5基光催化剂提供新途径。
Abstract:
CoxN (Co5.47N, Co2N) surface-modified Ta2N/Ta3N5 nanosheets were prepared by high-temperature nitridation technique, using Ta2O5@Ta3N5 as precursor, and Co3+ as modifier, which shows an ex-cellent visible light photoactivity for splitting water into hydrogen (59.2 umolg−1∙h−1), much higher than that of Ta2O5@Ta3N5 (21.75 umolg−1∙h−1). The composition, electronic structure, and charge carrier separation and transfer properties of the multi-heterostructures were modulated toward better photocatalytic performance by optimizing nitridation time. Construction of CoxN/Ta2N/Ta3N5 multiple heterojunctions significantly promotes the separation and migration of charge carriers. Subnitride Co5.47N producing a plasma resonance effect, and sub-bandgap behavior from delocalized Ta 5d of reduced Ta species and anion defect N-vacancies improved visible light absorption. Furthermore, the photocatalytic water analysis of hydrogen performance of the sample was im-proved. Constructing multi-heterostructures provides a promising avenue to design efficient and stable Ta3N5-based photocatalysts for solar water splitting.
1. 引言
利用太阳能光催化解水制氢是解决全球化石能源短缺和环境恶化问题的有效策略 [1]。开发高效稳定的光催化材料是制约光催化技术产业化应用的技术瓶颈。近年来,Ta3N5具有合适的带隙能(约2.1 eV),可见光的响应好,其导价带位置横跨H+/H2 (0 V vs NHE)和O2/H2O (1.23 V vs NHE)的氧化还原电势,无需外界偏压即可满足利用太阳光分解水对半导体材料带隙的要求,理论太阳能转氢效率(STH)高达15.9%,成为最具开发潜力的可见光分解水析氢候选材料之一。然而,Ta3N5存在光生载流子易复合、光生空穴易引发自氧化等缺限,导致光催化或光电催化析氢效率远低于5%,严重阻碍了其商业化应用进程 [2]。
据报道,通过Ar/H2高温还原处理,Ta3N5能够在Ta和Ta3N5界面生成低价的Ta2N和TaN,提高电导率促进电荷转移,并调控其电子结构,增加可见光吸收 [3] [4]。另据报道,钴基过渡金属化合物被广泛用于助催化剂,如磷化钴CoP和二硒化钴CoSe2用于HER助催化剂,氧化钴Co3O4或CoOx、氢氧化钴Co(OH)2、磷酸钴和硼酸钴用于OER助催化剂 [5] [6] [7] [8]。
本文以Ta2O5@Ta3N5为前驱物,以Co3+为修饰剂,利用高温氮化还原技术,将CoxN修饰在Ta2N/Ta3N5表面,制备CoxN/Ta2N/Ta3N5多重异质结纳米叶。通过氮化时间调控多重异质结的结构、组成及电荷分离和转移,提高其可见光催化解水析氢性能。该研究为构建高性能多重异质结构Ta3N5基光催化材料提供了新途径。
2. 实验部分
2.1. 试剂与仪器
试剂:TaCl5 (分析纯),湖南省华京粉体有限公司;甲醇(分析纯)、乙醇(分析纯),天津市光复科技有限公司;Co(NH3)6Cl3 (分析纯)、NaOH (分析纯),天津市科密欧化学试剂有限公司。
仪器:管式炉MXG1200-40S,上海微行炉业有限公司;氙灯350 W,上海蓝晟电子有限公司;X射线衍射仪Shimadzu XRD-6000,日本岛津公司;透射电子显微镜Tecnai G2TF20,美国FEI公司;紫外漫反射UV-2550型紫外光分光光度计,日本岛津公司;光电流测试CHI660E电化学工作站,上海辰华有限公司;产氢测试CEL-SPH2N,北京中教金源科技有限公司。
2.2. CoxN修饰Ta2N/Ta3N5光催化剂的制备
Ta2O5@Ta3N5前驱体的制备采用课题组前期报道的方法 [9],以TaCl5醇溶液为原料,采用水热法制备Ta2O5纳米颗粒,再经高温氮化制得。取1 mL 0.05 M Co(NH3)6Cl3溶液与1.5 mL 0.1 M的NaOH溶液加入30 mL蒸馏水于100 mL烧杯中混合溶液搅拌加热至黑色沉淀完全,加入0.295 g Ta2O5@Ta3N5,样品中Co:Ta的质量比为3 wt%,充分搅拌后抽滤,在80℃干燥12 h。取0.1 g样品平铺在2 × 5 cm石英舟中,置于管式炉中经1000℃分别氮化0.5 h、1 h、1.5 h、2 h制得CoxN修饰Ta2N/Ta3N5纳米光催化剂,分别记为Co-TN-0.5、Co-TN-1、Co-TN-1.5、Co-TN-2。
3. 结果与讨论
3.1. XRD分析
图1为Ta2O5@Ta3N5前驱体和Co-TN-X (X = 0.5、1、1.5、2)样品的XRD谱图。与Ta2O5@Ta3N5相比,修饰样品在38.7˚和43.7˚处出现两个新的衍射峰,分别为Ta2N (101)晶面(JCPDS 26-0985) [10] 和Co5.47N (111)晶面(JCPDS 41-0943) [11],其余峰归属于Ta3N5的特征峰的(020) (110) (023) (004) (113) (223)晶面(JCPDS 79-1533) [9]。随着氮化时间延长,缺氮化物Ta2N (101)的特征衍射峰峰增强,其强度几乎与Co-TN-2样品中Ta3N5 (113)晶面主峰的强度相当;Co5.47N (111)晶面也呈现类似的变化趋势。分析认为,NH3在1000℃分解产生H2,将Ta3N5表面还原生成了大量Ta2N和Co5.47N,这将有利于促进光生电子从Ta3N5转移至表面活性位。然而,Co2N (020)与Ta3N5的特征衍射峰重叠,难以确认。
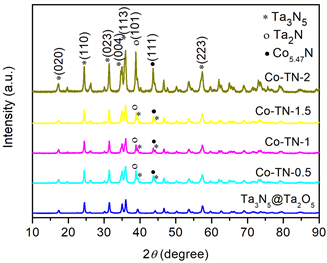
Figure 1. XRD patterns of Ta2O5@Ta3N5 and Co-TN-X (X = 0.5, 1, 1.5, 2)
图1. Ta2O5@Ta3N5和Co-TN-X (X = 0.5、1、1.5、2)样品的XRD谱图
3.2. TEM分析
图2为Ta2N/Ta3N5与Co-TN-1样品的TEM和HRTEM照片。由图2(a),图2(c)可见,Ta2N/Ta3N5与Co-TN-1样品均呈现不规则的纳米片结构,直径约为20~50 nm。由图2(b)可见,Ta2N/Ta3N5样品的晶格间距为0.243 nm和0.341 nm的衍射条纹,分别对应于Ta2N (002)和Ta3N5 (111)晶面。由图2(d)可见,Co-TN-1样品的晶格间距为0.207 nm、0.241 nm、0.264 nm、0.257 nm的衍射条纹,分别对应于Co5.47N (111)、Co2N (110)、Ta2N (100)、Ta3N5 (004)晶面,证实经高温氮化处理后样品形成了Co5.47N/Ta2N/Ta3N5和Co2N/Ta2N/Ta3N5多重异质结构。

Figure 2. (a) (c) TEM and (b) (d) HRTEM images of Ta2N/Ta3N5 and Co-TN-1 samples
图2. Ta2N/Ta3N5和Co-TN-1样品的(a) (c) TEM和(b) (d) HRTEM照片
3.3. UV-Vis分析
图3为Ta2O5、Ta2O5@Ta3N5、Co-TN-1样品的紫外可见吸收光谱。由图3可见,Ta2O5和Ta2O5@Ta3N5样品呈现出典型的半导体材料吸收特性,在321 nm和608 nm吸收边相应于3.86 eV和2.04 eV的带隙能。壳层Ta3N5的形成将吸收边拓展至可见光区,这归因于由N 2p轨道取代O 2p轨道提高了价带位置,从而降低了带隙能 [12]。与Ta2O5@Ta3N5前驱体相比,当CoxN与Ta2N/Ta3N5结合后,相应于Ta3N5的本征带隙吸收边消失,Co-TN-1样品在567 nm以上的可见光区表现出强吸收,这主要是由缺氮化物如Co5.47N产生了与贵金属类似的等离振子共振(LSPR)效应所致 [12];此外,低价Ta2N中离域的Ta 5d轨道及N3−空位引入了杂质能级也在一定程度上增强样品的可见光吸收 [13] [14]。
3.4. 光电流分析
在350 W氙灯模拟太阳光照射及施加0.2 V偏压条件下,Ta2O5@Ta3N5和Co-TN-X (X = 0.5, 1, 1.5, 2)样品的光电流如图4所示。由图4可知,样品光电流依次增加的顺序如下:Ta2O5@Ta3N5 < Co-TN-2 < Co-TN-0.5 < Co-TN-1.5 < Co-TN-1,当氮化时间为1 h制得样品的光电流最高约为24 uA∙cm−2。结果表明,构建CoxN/Ta2N/Ta3N5多重异质结能够明显提升光电流响应,促进光生电子和空穴分离和转移。
3.5. 可见光催化解水产氢活性
在可见光照射下,测试了Ta2O5@Ta3N5前驱体和Co-TN-1样品在20%甲醇水溶液中光催化解水产氢活性。Co-TN-1样品可见光催化产氢活性高达59.2 umol∙g−1∙h−1,远高于Ta2O5@Ta3N5前驱体的21.75 umol∙g−1∙h−1 [9]。因此,样品的可见光催化解水产氢活性的提高,应归因于CoxN/Ta2N/Ta3N5多重异质结构的形成改善了样品的可见光吸收、促进了光生载流子界面分离和迁移所致。
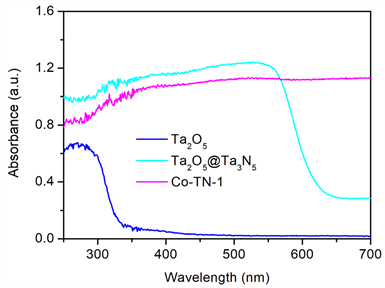
Figure 3. UV-vis absorption spectra of Ta2O5, Ta2O5@Ta3N5, Co-TN-1
图3. Ta2O5、Ta2O5@Ta3N5、Co-TN-1样品的紫外可见吸收光谱
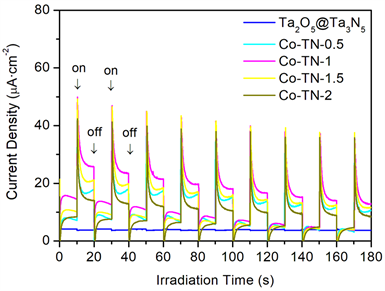
Figure 4. Photocurrent action spectra of Ta2O5@Ta3N5 and Co-TN-X (X = 0.5, 1, 1.5, 2)
图4. Ta2O5@Ta3N5和Co-TN-X (X = 0.5, 1, 1.5, 2)样品的光电流谱
4. 结论
利用高温氮化还原技术,Ta2O5@Ta3N5前驱体浸渍吸附3 wt% Co3+,经1000℃氮化1 h,将CoxN (Co5.47N、Co2N)修饰在Ta2N/Ta3N5表面,成功制备CoxN/Ta2N/Ta3N5多重异质结纳米片,其可见光催化解水产氢活性高达59.2 umol∙g−1∙h−1,远高于Ta2O5@Ta3N5前驱体的21.75 umol∙g−1∙h−1。构建CoxN/Ta2N/Ta3N5多重异质结构显著提升光电流,促进光生电子和空穴分离以及迁移;表面缺氮化物Co5.47N产生与贵金属类似的等离子共振效应,以及低价Ta2N中离域的Ta 5d和缺陷N3−空位引入的杂质能级共同作用,导致样品可见光吸收显著增强;上述因素协同作用,提高其可见光催化解水产氢活性。
NOTES
*通讯作者。