1. 引言
癌肉瘤是一种具有双相分化的恶性肿瘤,由癌及肉瘤两种成分构成,可发生于人体各个器官,其中发生于子宫的癌肉瘤(恶性苗勒管混合瘤)最为常见 [1]。发生在Vater壶腹部的原发性癌肉瘤罕见,目前检索到的国内外文献仅见7例报道 [2] [3]。在此,我们报道2例Vater壶腹部原发性癌肉瘤,其中一例腺癌成分伴肠母细胞分化国内外尚未见报道,并对其临床病理特征进行分析并综合文献复习,以提高临床及病理医师对此类肿瘤的认识。
2. 资料与方法
2.1. 病例选择
回顾性分析2011年1月~2020年6月青岛大学附属医院诊治的2例Vater壶腹部原发性癌肉瘤,复习相关临床病史、影像学及实验室检查和病理诊断等资料,并对患者进行随访观察。
2.2. 方法
手术标本均经10%中性福尔马林溶液固定,常规石蜡包埋,4 μm厚连续切片,分别行常规HE染色和免疫组织化学染色。免疫组织化学染色使用罗氏VENTANA全自动免疫组织化学染色机,均设置阳性及阴性对照,按照说明书进行操作。所用一抗工作液包括细胞角蛋白(CKpan、CK7、CK20、CK19)、波形蛋白(Vimentin)、磷脂酰肌醇蛋白聚糖3 (GPC-3)、尾型同盒转录子-2 (CDX-2)、绒毛蛋白(Villin)、突触素(Syn)及嗜铬素A (CgA),购自福州迈新生物技术有限公司;婆罗双树样基因(SALL4)及甲胎蛋白(AFP)购自北京中杉金桥公司。HE及免疫组化切片均由2位高年资病理医师复检。
3. 结果
3.1. 临床资料
例1,女性,65岁。因餐后腹胀、嗳气4月余入院。上腹部CT动态增强扫描及超声胃镜(图1)均提示十二指肠壶腹部占位。术前行十二指肠乳头病变活检,考虑为肉瘤。实验室检查总胆红素24.31 μmol/L (正常3.00~22.00)、总胆汁酸17.63 μmol/L (正常0~12)、谷丙转氨酶731.00 U/L (正常7~40)、谷草转氨酶980.00 U/L (正常13~35)、谷氨酰转肽酶549.00 U/L (正常7~45)、碱性磷酸酶296.00 U/L (正常50~135)及乳酸脱氢酶770.00 U/L (正常120~250)等显著升高,CEA、AFP、CA199均在正常范围内。入院后行“胰十二指肠切除术”,术后标本于Vater壶腹部见3 × 2 cm大小质硬肿块,切面灰褐色,肿物周围未触及肿大淋巴结。例2,女性,48岁。因上腹痛2月,小便黄1月入院。上腹部CT动态增强扫描提示胰腺壶腹区占位,考虑肿瘤。实验室检查总胆红素38.91 μmol/L (正常3.00~22.00)、直接胆红素25.24 μmol/L (正常0~8.0)、总胆汁酸218.40 μmol/L (正常0~12)、谷丙转氨酶157.00 U/L (正常7~40)、谷草转氨酶85.00 U/L (正常13~35)、谷氨酰转肽酶711.00 U/L (正常7~40)及碱性磷酸酶219.00 U/L (正常50~135)等显著升高,CA125197.00 U/ml (正常0~35)、CA19985.34 U/ml (正常0~39)显著升高,AFP、CEA在正常范围内。入院后行“胰十二指肠切除及后腹膜淋巴结清扫术”,术后标本于Vater壶腹部扪及质硬肿块,大小约2.5 × 2 cm,周围未见异常肿大淋巴结。
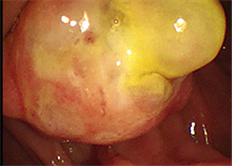
Figure 1. Case 1 Ultrasound Endoscope: space occupying lesion in the ampulla
图1. 例1超声内镜:壶腹部占位性病变
3.2. 镜下观察
两病例中均可见癌和肉瘤两种成分。第一例癌成分中肿瘤细胞排列成腺管状、筛状、巢状或单个癌细胞浸润,细胞核大深染,核仁易见,可见病理性核分裂象,胞浆丰富嗜酸性(图2);第二例癌成分中肿瘤细胞排列成管状、乳头状或腺样,大部分肿瘤细胞呈柱状,胞浆透亮,胞核圆形或柱状,部分可见核仁,类似于早期胎儿肠上皮(图3),少部分肿瘤细胞胞浆嗜酸性,并见多灶坏死。两病例中肉瘤成分均可见呈条束状、席纹状或不规则排列的长梭形核细胞、奇异形核细胞及多核瘤巨细胞(图4);第一例可见肿瘤性骨样基质及骨样细胞,呈花边状浸润性生长(图5,左),第二例可见少量肿瘤性软骨样基质及陷窝细胞(图5,右),分别具有骨肉瘤及软骨肉瘤特征。两病例均无血管、淋巴管及周围神经侵犯,无区域淋巴结转移及远处转移。
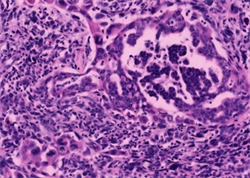
Figure 2. Case 1 the components of carcinoma cells arranged in glandular, cribriform, nest-like or single cancer cell infiltration, with large hyperchromatic nuclei, visible nucleoli, pathologic mitosis, and abundant eosinophilic cytoplasm (HE × 400)
图2. 例1癌成分肿瘤细胞排列成腺管状、筛状、巢状或单个癌细胞浸润,细胞核大深染,核仁易见,可见病理性核分裂象,胞浆丰富嗜酸性(HE × 400)
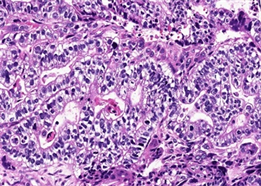
Figure 3. Case 2 the components of carcinoma cells arranged in tubulopapillary or glandular, composed of columnar neoplastic cells with clear cytoplasm, resembling early fetal gut epithelium (HE × 400)
图3. 例2癌成分肿瘤细胞排列成管状乳头状或腺样,肿瘤细胞呈柱状,胞浆透亮,类似于早期胎儿肠上皮(HE × 400)
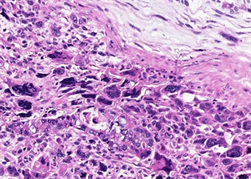
Figure 4. Two cases the components of sarcoma showed spindle cells, singular cells and multinucleated giant cells (left, case 1, right, case 2; HE × 400)
图4. 两病例肉瘤成分可见长梭形核细胞、奇异形核细胞及多核瘤巨细胞(左,例1,右,例2;HE × 400)
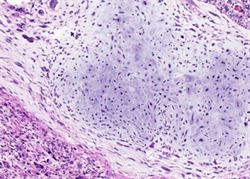
Figure 5. Case 1 showed neoplastic osteoid matrix and osteoid cells; Case 2 showed neoplastic chondroid matrix and lacunae cells (left, case 1, right, case 2; HE × 200)
图5. 例1可见肿瘤性骨样基质及骨样细胞;例2可见肿瘤性软骨样基质及陷窝细胞(左,例1,右,例2;HE × 200)
3.3. 免疫组织化学
两病例中癌成分均表达CK,不表达Vimentin;肉瘤成分均表达Vimentin,不表达CK (图6、图7)。第一例中癌成分同时表达CK7、CK19,不表达CK20、CDX-2;第二例中癌成分表达CK20、CDX2、Villin,CgA灶状阳性,Syn阴性,其中胞浆透亮的腺癌柱状细胞同时表达SALL4、GPC-3和AFP (图8)。
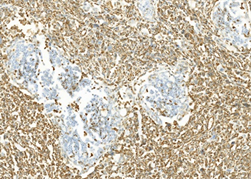
Figure 6. Case 1 the components of carcinoma were immunopositive for CK, losing of Vimentin protein expression; The components of sarcoma were immunopositive for Vimentin, losing of CK protein expression (left, CK, right, Vimentin; HE × 200)
图6. 例1癌成分均表达CK,不表达Vimentin;肉瘤成分均表达Vimentin,不表达CK (左,CK,右,Vimentin;HE × 200)
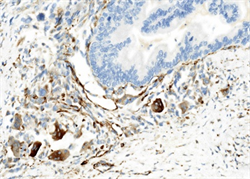
Figure 7. Case 2 the components of carcinoma were immunopositive for CK, losing of Vimentin protein expression; The components of sarcoma were immunopositive for Vimentin, losing of CK protein expression (left, CK, right, Vimentin; HE × 400)
图7. 例2癌成分均表达CK,不表达Vimentin;肉瘤成分均表达Vimentin,不表达CK (左,CK,右,Vimentin;HE × 400)
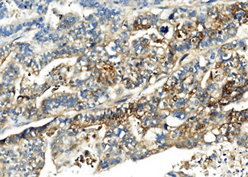
Figure 8. Case 2 the components of adenocarcinoma with enteroblastic differentiation were positive for SALL4, GPC-3 and AFP (left, SALL4, middle, GPC-3, right, AFP; HE × 400)
图8. 例2伴肠母细胞分化的腺癌成分表达SALL4、GPC-3和AFP (左,SALL4,中,GPC-3,右,AFP;HE × 400)
3.4. 病理诊断
例1,(Vater壶腹部)癌肉瘤,肿瘤构成于中–低分化腺癌及多形性未分化肉瘤,其中肉瘤成分中部分区域呈骨肉瘤分化。例2:(Vater壶腹部)癌肉瘤,肿瘤成分大部分为中–低分化腺癌,部分区域腺癌伴肠母细胞分化;肉瘤成分构成于多形性未分化肉瘤,部分区域呈软骨肉瘤分化。
3.5. 随访
两例均行胰十二指肠切除手术,术后予以支持治疗,未行其它治疗措施。例1术后随访28个月,状态良好,无复发和转移;例2术后随访3个月,期间曾出现药物性皮炎,无其他不良反应,至今无瘤生存。
4. 讨论
癌肉瘤是一种具有双相分化的恶性肿瘤,由癌及肉瘤两种成分构成,可发生于人体各个器官 [1],在女性生殖道、上消化道,肺脏、泌尿道、头颈部、乳腺、甲状腺等部位均可发生。发生在Vater壶腹部的癌肉瘤罕见,目前检索到的国内外文献仅见7例报道 [2] [3]。这7例病例中癌成分大部分以腺癌为主,一例可见鳞状细胞癌成分,另一例可见神经内分泌癌成分;肉瘤成分均以未分化梭形细胞肉瘤为主,一例可见骨肉瘤分化。发生在壶腹部的癌肉瘤临床上病人常以腹痛、腹胀、尿黄等主诉入院,实验室检查胆红素、胆汁酸、碱性磷酸酶等梗阻性黄疸指标升高,肉眼观察大多为息肉样或隆起样肿物,易造成早期梗阻症状。Matsunou等 [4] 在1994年首次报道了两例胃的伴肠母细胞分化的腺癌,WHO (2019)消化系统肿瘤分类 [5] 中提及了此类肿瘤,但并未作为单独亚型列出。近几年陆续有研究报道其临床病理及分子学特征。我们报道的2例Vater壶腹部癌肉瘤中,其中一例癌成分构成于伴肠母细胞分化的腺癌则在国内外首次报道,2例病例中的肉瘤成分均伴有异源性成分,分别表现为骨肉瘤和软骨肉瘤分化。
目前关于癌肉瘤的组织起源尚不明确,主要有两种假说 [6],一种是多克隆假说,即癌肉瘤是由两个或多个上皮和间充质来源的干细胞恶性转化后构成的碰撞瘤;另一种则是单克隆假说,即癌细胞和肉瘤细胞来源于一个单一的多能干细胞,其恶性转化后沿着不同的上皮和间充质途径进行分化。癌肉瘤尚不明确的组织起源给病理诊断和临床治疗带来了困惑。对于癌肉瘤至今没有给出一个明确的定义,但一般认为癌肉瘤是一种伴有癌和肉瘤成分(如恶性软骨、骨或横纹肌)构成的双向分化的混合性恶性肿瘤。然而,近年来许多研究认为癌肉瘤中的肉瘤成分可能是上皮来源,研究 [7] 提示上皮细胞–间充质转化(Epithelial-Mesenchymal Transition, EMT)途径的激活在上皮成分转化成肉瘤或肉瘤样细胞的过程中起重要作用,包括塑形成纤维细胞样,增强细胞运动能力,并上调间质标志蛋白。当上皮细胞向间质细胞转化时,其形态由卵圆形转变为纺锤形。目前EMT途径激活被认为与癌肉瘤的发生、发展密切相关,将其称为肉瘤样癌或癌伴肉瘤样分化更为合适,但是目前与壶腹部癌肉瘤研究相关的文献报道较少。癌肉瘤中癌成分常见的有腺癌、鳞状细胞癌或未分化癌,肉瘤成分中多为多形性未分化肉瘤,并可见异源性成分,如骨肉瘤、软骨肉瘤、平滑肌肉瘤、横纹肌肉瘤等。我们报道的2例Vater壶腹部癌肉瘤中的癌和肉瘤成分均符合上述特征。
伴肠母细胞分化的腺癌最早在胃腺癌中报道,目前在肠腺癌、胆囊腺癌等肿瘤中已有报道。Kenichiro Furukawa等 [8] 报道一例食管胃交界处的癌肉瘤,其中腺癌成分伴有肠母细胞分化。Koko Mitsuma等 [9] 报道一例发生在壶腹部的肿瘤,肿瘤构成于伴肠母细胞分化的腺癌和神经内分泌癌。WHO (2019)消化系统肿瘤分类 [5] 中提到,伴肠母细胞分化的胃腺癌是一种由胞浆透亮的类似于早期胎儿肠上皮的柱状肿瘤细胞构成的肿瘤,排列成管状乳头状,常有SALL4、GPC-3和AFP中至少一种的表达。SALL4是在胚胎干细胞中表达的锌指转录因子,可在生殖细胞和胎儿肠道细胞中表达,它是胚胎多能性干细胞的主要调节因子以及其他多能性相关的转录因子。AFP是由胚胎肝细胞,胚胎卵黄囊细胞和胎儿肠道细胞合成的哺乳动物胚胎血清蛋白,一般认为AFP的产生是肿瘤细胞逆分化为产AFP胎儿细胞的结果。GPC-3是细胞表面硫酸乙酰肝素蛋白聚糖之一,除胎盘和胎肝之外,在正常组织中不表达,同时GPC-3也是一种癌胚蛋白,可在肝细胞肝癌,恶性黑色素瘤等多种肿瘤中表达。诊断伴肠母细胞分化的腺癌需要结合形态学(胞浆透亮的柱状细胞排列成管状、乳头状结构)及至少SALL4、GPC-3和AFP免疫组化中的一种阳性 [4] [10]。本研究中的其中一例病例中,腺癌成分形态学特点及免疫组化表达均符合伴肠母细胞分化的腺癌诊断。伴肠母细胞分化的腺癌其发生可能与TP53、DNMT3A、SMAD4等基因突变有关,常有HER2基因的扩增,文献报道 [11] [12] 其具有很强的血管侵犯和肝转移特性,常常提示预后不良。因此认识伴肠母细胞分化的腺癌对临床治疗和判断预后可能有一定的提示意义。
发生在壶腹部的癌肉瘤包括癌和肉瘤两种恶性肿瘤成分,于光镜下鉴别诊断困难时,需行免疫组织化学染色,其中癌成分免疫组化表现为CK、EMA阳性,Vimentin阴性,肉瘤成分免疫组化表现为Vimentin阳性,CK、EMA阴性。癌肉瘤的镜下形态主要需与肉瘤样癌鉴别,肉瘤样癌属于上皮源性肿瘤,本质上属于分化差的癌。形态上可全部为单一的肉瘤样梭形或多形细胞;或具有癌与肉瘤样两种形态,以肉瘤样形态为主。在典型病例中,癌和肉瘤样形态具有移行过渡,免疫组化染色癌样与肉瘤样成分均表达上皮性标记物CK等,肉瘤样成分同时也表达间叶性标记物Vimentin等。本研究中两例病例癌成分均表达CK,不表达Vimentin;肉瘤成分均表达Vimentin,不表达CK,则支持癌肉瘤的诊断。
Vater壶腹部癌肉瘤是一种高度侵袭性肿瘤,预后较差,由于其罕见性,文献报道的壶腹部癌肉瘤主要以手术切除治疗为主并辅以化疗、放疗,但是效果并不显著,患者大多死于肿瘤复发和远处转移。由于本研究纳入的2例患者均行胰十二指肠切除术,术后分别随访28个月、3个月,均无复发转移,但两例病例由于术后时间较短,还需进一步随访观察。
同意书
本研究已获得病人的知情同意。