1. 引言
酚类化合物具有较大的毒性,并且广泛存在于工业废水中,不仅污染自然水体还会危害人体健康,生物和物理等方法难以高效处理。通过活化过硫酸盐产生硫酸根自由基(SO− 4∙)和羟基自由基(∙OH)的高级氧化技术(AOPs)是一种高效、快速的技术手段,在处理难降解废水领域受到广泛关注 [1] [2] [3] [4] [5]。
过硫酸盐产生SO− 4∙和∙OH的方式主要包括紫外线、热活化和过渡金属活化等 [6] [7] [8] [9]。紫外线和热活化能耗较高,单独的活性炭活化效果不理想。因此,许多研究都集中在过渡金属掺杂碳材料上,不仅能克服金属催化剂产生的二次污染,还因碳质材料与金属组分偶联形成复合结构,获得新的物理化学性质,形成更多的催化位点以增强碳基材料催化性能 [10] - [16]。
聚苯胺因其特殊的性质,可以成为良好的金属掺杂碳基体,常以过硫酸铵作为氧化剂氧化聚合苯胺,产生的无机副产物后处理过程复杂。H2O2是一种绿色氧化剂,唯一副产物为H2O,可简化后处理工序。但单一H2O2作为氧化剂反应速率慢,因此利用类芬顿氧化法,通过在反应体系中加入适量钴源(如四氧化三钴),促进H2O2分解产生具有强氧化性的羟基自由基氧化聚合苯胺,同时体系中的钴离子可以和聚苯胺中的氮原子配位,得到负载钴的聚苯胺,经过碳化即可得到钴掺杂碳材料。利用其活化PMS氧化降解含苯酚模拟废水,探究材料合成中钴添加量,降解体系中PMS投加量、催化剂投加量以及体系初始pH对降解效果的影响。
2. 实验部分
2.1. 实验试剂
苯胺、过硫酸氢钾(PMS)、过氧化氢、四氧化三钴、盐酸、乙醇、橙黄G(OG)、苯酚等均购自国药集团化学试剂有限公司,所有药品规格均为分析纯级。
2.2. 样品的制备
称取一定量的四氧化三钴,溶于5 mL浓盐酸中,加入30 mL去离子水,搅拌均匀后加入1 mL苯胺,之后滴加2 mL双氧水(30%),置于4℃~10℃条件下反应24 h,离心分离沉淀,用乙醇和去离子水洗涤三次得到墨绿色固体。取出一定量此前驱体在N2氛围下,以5℃/min的速率升温至800℃煅烧1 h,得到Co-NC材料。
2.3. 样品的表征
催化剂物相结构采用X射线粉末衍射仪(XRD)测定(Bruker公司,型号D8)。样品形貌和粒径采用扫描电子显微镜(SEM) (日本电子株式会社,型号JSM5510LV)观察。
2.4. 催化性能测试
称取一定量催化剂到有50 mL 25 mg/L污染物(如OG、苯酚)溶液的两口烧瓶中,在25℃水浴、600 rpm条件下预吸附30 min,然后加入一定量的PMS,于不同时刻取样通过0.22 µm滤膜过滤后,所用紫外-可见光分光光度仪为UV-6100 (上海美谱达仪器有限公司),OG检测波长分别为475 nm。
苯酚采用高效液相色谱检测(Thermo scientific公司,型号:UltiMate 3000),苯酚检测条件 [17] 为:流动相为V (H2O): V (甲醇) = 40:60,流速:0.8 mL/min,检测波长:271 nm,柱温:30℃,进样量:20 µL,色谱柱规格:C18,5 µm Analytical 4.6 × 150 mm。
2.5. 钴掺杂碳材料催化降解苯酚的循环使用性能
对反应后的溶液离心分离收集固体(5000 rpm离心5 min),对材料进行简单洗涤后烘干,按照1.4催化性能测试进行循环使用性能测试。
2.6. 污染物脱除率测定方法
通过吸光度值得出剩余污染物溶液的浓度并计算污染物脱色率或脱除率,按式(1)计算溶液的脱除率,本文也用C/C0表示污染物去除率。
(1)
式中C0和C分别为污染物的初始和残余浓度值(mg/L)。
3. 结果与讨论
3.1. 材料的表征分析
Co掺杂碳材料的表征如图1所示,图1(a)是材料的XRD,可以看到分别有两个宽峰,可以归属为类石墨结构的(002)和(101)晶面衍射峰,从XRD可以看出材料的石墨化程度不高,主要是无定型碳。图1(b)为材料的SEM,可以看出材料的整体形貌为无规则的棒状。
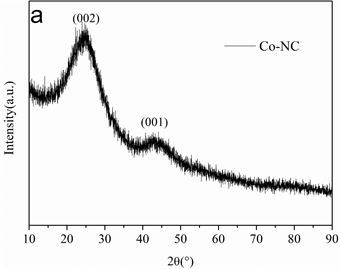
Figure 1. XRD (a) of Co-NC materials, SEM (b) of Co-NC materials
图1. Co-NC材料的XRD (a),Co-NC材料的SEM(b)
3.2. 不同钴添加量对Co-NC催化降解性能的影响
选择阴离子染料OG作为模拟有机废水,考察不同钴添加量合成的Co-NCn (n = 0.01 g、0.03 g、0.05 g、0.07 g)材料活化PMS的催化降解活性,实验结果如图2所示,当钴添加量为0.01 g和0.03 g时,Co-NCn材料的催化PMS的能力有限,20 min只能降解50%~60%的OG (25 mg/L),循环三次没有明显活性损失。但随着钴添加量的增加,Co-NCn材料活化PMS降解OG的去除率不断增加,在添加量为0.05 g时,Co-NC 0.05材料可以催化PMS在20 min内完全降解25 mg/L的OG,且循环使用三次没有明显活性损失。
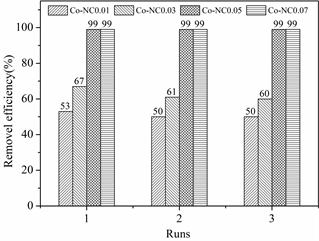
Figure 2. Effect of cobalt addition on degradation performance
图2. 钴添加量对降解性能的影响
合成材料体系中的钴离子可以和聚苯胺中的氮原子配位,得到负载钴的聚苯胺,高温碳化后得到的Co掺杂碳材料,碳材料原有的理化性质发生改变,增加了新的活性位点,从而提高了材料的催化性能。但是在添加量达到一定后,持续添加不会进一步提高降解性能,可能是因为碳材料掺杂金属已经达到饱和,进一步添加也未能形成新的活性位点。
3.3. 催化性能测试
本节选择苯酚作为模型有机污染物,考察Co掺杂碳材料催化PMS的催化降解活性。30 min内,单独PMS不能降解苯酚。只加入Co掺杂碳材料,30 min内达到吸附平衡,吸附率为6%。当同时加入Co掺杂碳材料和PMS时,30 min内苯酚降解率达到99%以上,证明Co掺杂碳材料的加入,促进PMS的活化分解,产生大量的强氧化性自由基,从而有效降解水中苯酚(图3)。
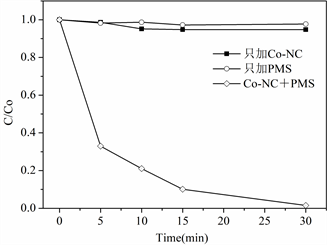
Figure 3. Co-NC catalyzed the degradation of phenol
图3. Co-NC催化降解苯酚
3.4. PMS浓度对降解性能的影响
在室温,pH = 7.0条件下,控制苯酚初始浓度不变(25 mg/L),改变PMS添加量,探究对PMS和Co掺杂碳材料对降解效果的影响。结果如图4所示,当PMS和苯酚的物质的量比值为4:1时,30 min苯酚降解率为30%;当PMS与苯酚物质的量比从12:1增加到30:1时,苯酚的降解率有所提高,降解率从40%增加到99%,当继续增大至50:1时,苯酚降解率反而降低。这可能是因为PMS过量从而产生了过量的硫酸根自由基,并发生淬灭反应,使得硫酸根自由基有效浓度降低,导致苯酚降解效果降低。
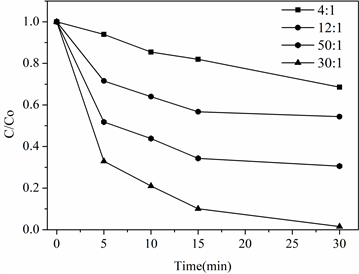
Figure 4. Effect of PMS concentration on degradation performance
图4. PMS浓度对降解性能的影响
3.5. 催化剂添加量对降解性能的影响
在室温,pH = 7.0条件下,控制苯酚初始浓度不变(25 mg/L),改变催化剂投加量对苯酚降解效果的影响如图5所示。在Co掺杂碳材料投加量为10 mg时,30 min内苯酚降解率为60%,当投加量增加到25 mg时,苯酚降解率达到99%;当进一步提高改性碳材料投加量至40 mg,苯酚降解效果并没有进一步提高,可能是因为低浓度下,Co掺杂碳材料的增加,能提供更多的活性位点,使得PMS活化分解效果显著提升,从而使得苯酚降解效果增加。当过量的Co掺杂碳材料被加入时,短时间会产生大量自由基,导致自由基之间相互淬灭,导致苯酚降解效果不会进一步提升。
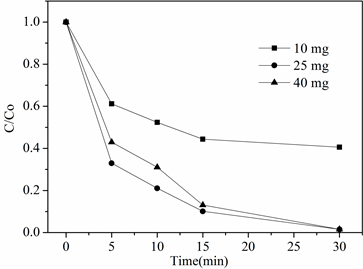
Figure 5. Effect of catalyst addition on degradation performance
图5. 催化剂添加量对降解性能的影响
3.6. 溶液初始pH对降解性能的影响
溶液初始pH对苯酚降解效果的影响如图6所示。当溶液pH = 7.0时,30 min苯酚降解率为99%;当pH = 5.0时,苯酚降解率为65%;当pH = 9.0时,苯酚降解率为60%,而pH = 11.0时,苯酚降解率下降至50%。实验结果表明,在中性环境下降解效果最好,pH值得增大或减小都会在一定程度上影响苯酚的降解效果。pH在3~6时,反应体系中是以SO− 4∙和为主,在酸性和中性环境中SO− 4∙的含量和反应速率都要高于碱性环境,并且pH在9~11时共存∙OH和SO− 4∙还会相互反应,减少体系中自由基的有效浓度,导致降解效果降低。
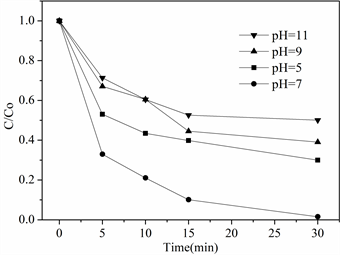
Figure 6. Effect of initial pH of solution on degradation performance
图6. 溶液初始pH对降解性能的影响
3.7. 材料的循环性能
由于Co掺杂碳材料对催化降解苯酚的优异性能,以苯酚为目标污染物考察了Co掺杂碳材料的循环使用性能,如图7所示,经过5次循环使用,钴改性碳材料催化降解性能基本保持不变,在循环第四次和第五次时催化活性有所降低,第四次循环降解率为93%,第五次循环降解率为90%。
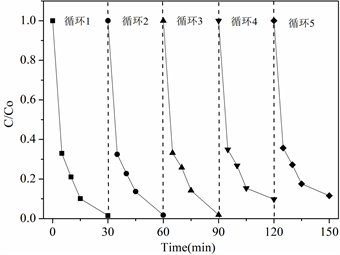
Figure 7. Cyclic performance of catalyst
图7. 催化剂的循环性能
4. 结论
利用类芬顿反应,以苯胺为原料、H2O2为氧化剂原位合成Co掺杂碳基环境材料,系统探究Co掺杂材料活化PMS降解苯酚的性能。
Co掺杂量为0.1 mmol,合成的Co掺杂材料活化PMS效果最好;当Co掺杂碳材料投加量为25 mg、PMS与苯酚的物质的量比值为30:1、pH = 7.0、30 min内可以完全降解苯酚;材料重复使用5次,性能有所下降,在循环使用第五次时,30 min内对苯酚的降解率为90%。
参考文献
NOTES
*通讯作者。