1. 引言
金属可以与水发生放热反应并产生氢气,因此金属/水反应可以应用于制氢技术 [1] - [7] ,也可以应用于水下推进系统 [8] [9] [10] ,反应过程中产生的高温氢气伴随着巨大能量能够推动水下航行体运动。Al粉的能量密度高,且价格低廉、与水反应的产物无毒,单质Al粉在水下推进系统中的应用引起了大量关注 [11] [12] ,Al与水的反应已被广泛研究 [13] - [19] 。Al粉与水的反应存在难以启动与持续的问题,因为Al粉颗粒在空气中极易被氧化,表面会形成一层致密的氧化膜,这层氧化膜阻碍了内部Al和水分子的接触与反应 [20] 。因此研究人员探索了一些方法来促进铝/水反应,比如将铝在碱性溶液中反应 [6] [21] ,使用铝基复合材料 [22] [23] [24] 等。
Mg与水反应具有反应启动温度低、反应效率高的优点,已被广泛应用于水反应产氢领域 [25] - [30] 。Uda等人 [25] 制备的高活性纳米Mg粉在室温下即可与水剧烈反应。Liu等人 [26] 分别将Mg粉与AlCl3、KCl、NaCl和MgCl2进行混合球磨,并研究其水反应性能,发现AlCl3与Mg的球磨混合物的水反应效率最高。
基于此,研究人员提出Al粉中添加Mg是一种提高水反应活性的有效方法 [11] [31] [32] [33] 。Zou等人 [31] 对Mg-Al合金与海水的反应进行了研究,发现高能球磨后的Mg-Al颗粒更细小,水反应活性更高。Kozin等人 [32] 研究了Al-Mg-Bi合金水反应时铝和镁的相互作用机理,证实Bi可以起到活化剂的作用,使铝和镁具有高反应活性。Yang等人 [33] 进行了Mg含量为20 wt.%的Al-Mg混合粉末在高温水蒸气中的热重实验,并用质量增重来衡量反应效率。试验结果表明,Al-20Mg混合粉末与Al粉相比,反应效率从13.52%提高到了50.51%,Al-20Mg的水反应产物包含Al2O3、MgO与MgAl2O4,并存在部分剩余单质Al。
本文采用高温气雾化法分别制备了Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu(wt.%)三种合金燃料粉末,其中Li的水反应活性很高,Eu是一种高活性的稀土元素。然后使用高压反应釜实时监测不同合金粉末水反应的环境温度和压强,并根据反应启动温度、反应时间、产气量与产氢效率综合评价其水反应活性和性能,并对反应产物进行了表征。
2. 实验部分
2.1. 试剂与仪器
所用原材料包括Al锭(99.9%纯)、Mg锭(99.9%纯),Li锭(99.9%纯)与Eu锭(99.9%纯),均购自徐州华中铝业公司。合金燃料粉末通过高温气雾化法制备,雾化介质为高纯氩气,雾化压力5 MPa。所用单质Al粉购自江苏威拉里新材料科技有限公司。所有粉末均过325目筛(<45 μm)。
2.2. 实验过程
使用场发射扫描电子显微镜(SEM, Nova NanoSEM 450)表征合金粉末及水反应产物的表面形貌。使用带有Cu Kα射线的X射线衍射仪(XRD, PANalytical B.V.)表征合金粉末及水反应产物的物相组成,衍射角范围是10˚~90˚。
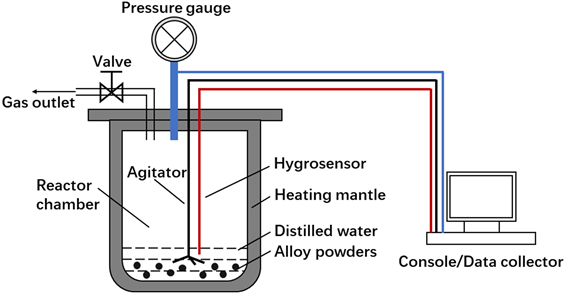
Figure 1. Diagram of high pressure reactor equipment
图1. 高压反应釜设备示意图
为了研究不同种类合金粉末高温高压条件下的水反应特性,使用高压反应釜进行水反应实验。图1为高压反应釜设备示意图。具体实验步骤如下:使用高精度电子天平准确称量一定质量的合金粉末样品,并置于反应釜中,使用移液枪准确量取30 mL蒸馏水(过量)并加入反应釜中,蒸馏水与样品物质的量之比为10:1。将反应釜盖子拧紧,气阀关闭,以保证气密性。启动设备电源,设置炉温为500℃,搅拌速率为500 r/min,开始加热和搅拌。加热炉温度升高的同时将热量传递给内部的反应釜,该设备可以自动监测整个反应过程中的加热炉温度、反应釜温度和反应釜压强。反应结束后停止加热和搅拌,待反应釜冷却至室温后将其打开并收集反应产物,并对设备记录的温度、压强数据进行处理和分析。为了排除反应釜内过量水对体系温度及压强的影响,设计了一个空白对照组,即在反应釜中只加入30 mL蒸馏水,在同样参数下进行实验,并将此空白实验与水反应实验进行比较。
3. 结果与讨论
3.1. Al基合金粉末的物相组成与表面形貌
高温气雾化法制备的三种合金粉末的XRD图谱如图2所示。Al-40Mg合金粉末由Al3Mg2和Al12Mg17两种物相组成,这符合Al-Mg二元合金相图的规律。Al-37Mg-3Li和Al-37Mg-3Eu合金粉末的衍射峰均分别对应Al3Mg2和Al12Mg17两种物相,没有检测到明显的含Li或Eu的物相,这是由于Li元素和Eu元素的含量太低,低于XRD设备的监测下限。
Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu合金粉末的SEM照片显示在图3中。显然,三种合金粉末颗粒的球形度很高,表面非常光滑,并且分散性良好,没有明显的团聚,表明这三种合金粉末具有良好的流动性。图4为Al-37Mg-3Eu合金粉末单颗粒的SEM照片及EDS面扫描结果。从图中可以看出,Al-37Mg-3Eu合金粉末颗粒表面均匀分布着Al、Mg、Eu三种元素,其中Al、Mg的含量较高,Eu含量偏低,采用气雾化法所制备的合金粉末中三种金属元素熔合得较好。
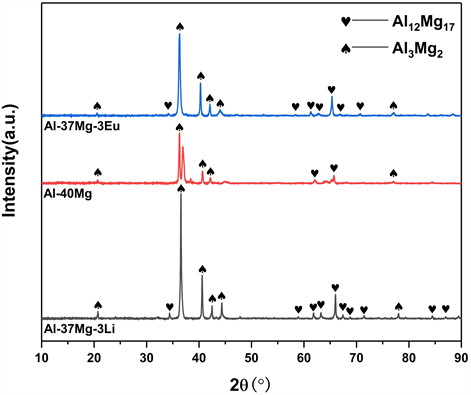
Figure 2. XRD patterns of Al-40Mg, Al-37Mg-3Li and Al-37Mg-3Eu alloy powders
图2. Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu合金粉末的XRD图谱

Figure 3. SEM images of Al-40Mg, Al-37Mg-3Li and Al-37Mg-3Eu alloy powders: (a) Al-40Mg, (b) Al-37Mg-3Li, (c) Al-37Mg-3Eu
图3. Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu合金粉末的SEM照片:(a) Al-40Mg,(b) Al-37Mg-3Li,(c) Al-37Mg-3Eu
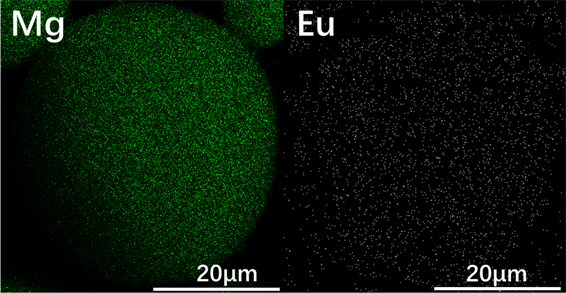
Figure 4. SEM image and EDS scanning results of single particle Al-37Mg-3Eu alloy powder
图4. Al-37Mg-3Eu合金粉末单颗粒的SEM照片及EDS面扫描结果
3.2. Al基合金粉末的水反应性能
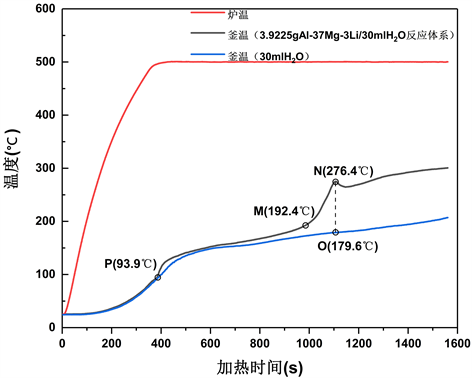
Figure 5. Temperature-time curve of reaction of Al-37Mg-3Li alloy powder with water
图5. Al-37Mg-3Li合金粉末与水反应的温度–时间曲线
图5是Al-37Mg-3Li与水反应的温度–时间曲线。图中红色曲线是加热炉的温度变化曲线,加热炉的温度快速升高达到设定值后,其温度便保持恒定;黑色曲线是反应釜的温度变化曲线,即表示了水反应体系的温度变化;蓝色曲线是相同条件下,仅在反应釜中加入蒸馏水时的反应釜温度变化曲线(也即空白对照组)。将Al-37Mg-3Li/H2O反应体系的温度变化趋势与空白对照组的温度变化趋势进行对比,分析水反应体系的反应过程和热量变化。
图5显示,第一阶段是P点之前,Al-37Mg-3Li/H2O的T-t曲线与H2O的T-t曲线基本吻合,此时反应还未进行。第二阶段从P点开始,Al-37Mg-3Li/H2O的T-t曲线开始偏移H2O的T-t曲线,此时反应开始进行,反应放热使温度升高;反应进行到M点时,温度开始急剧升高,此时Al-37Mg-3Li与H2O的反应剧烈进行,直至N点,温度达到一个峰值,随后温度变化平缓,与空白组的温度变化趋势相近,说明在N点之后反应体系没有贡献出额外的放热量,N点处反应基本结束。第三阶段即为N点以后,反应结束。
从图5的水反应T-t曲线上可以提取出3个重要的特征参数:
1) 反应起始温度:图5中的P点,93.9℃。在该温度点处,反应开始启动。对金属/水反应体系而言,反应起始温度越低越有利。
2) 反应时间:图5中的N点与P点所间隔的时间即为反应时间。反应时间越短,则反应放热越集中,金属/水反应活性越强。
3) 反应放热量:当水反应体系处于温度峰值N点时,此时,蒸馏水对照组的温度点为O点,而N点之后反应结束,因此|TN-TO|(ΔT)可以表示反应放热量对温度升高的贡献,ΔT越大,则反应放热量越大。
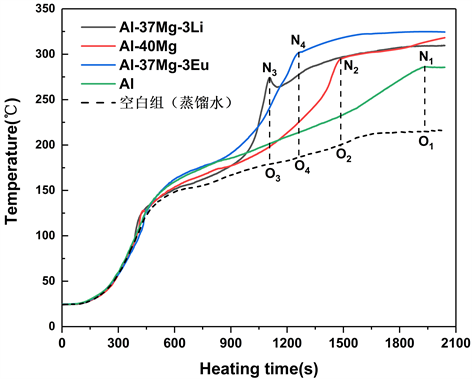
Figure 6. Temperature-time curve of water reaction of Al, Al-40Mg, Al-37Mg-3Li and Al-37Mg-3Eu powders
图6. Al、Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu粉末的水反应温度–时间曲线
图6是在同一水燃比(10:1)、相同量的蒸馏水(30 mL)条件下,Al、Al-37Mg-3Li、Al-40Mg与Al-44Mg-1Eu四种粉末样品与水反应的温度–时间曲线。提取四种粉末的水反应启动温度、反应时间、反应温升ΔT等反应特征参数,结果列于表1。

Table 1. Water reaction characteristic parameters of Al, Al-40Mg, Al-37Mg-3Li and Al-37Mg-3Eu powders
表1. Al、Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu粉末的水反应特征参数
由表1可知,四种粉末中水反应启动温度最低的是Al-37Mg-3Li,ΔT最大的是Al-37Mg-3Eu,并且这两种合金粉末的水反应时间都较短。与单质Al粉相比,Al-40Mg的水反应的启动温度降低,反应时间也极大缩短,Mg的加入有利于提高合金粉末的水反应活性。Al-37Mg-3Li比Al-40Mg的反应启动温度提前了很多,且反应时间进一步缩短,反应也导致了更大的温度升高,这说明活泼金属Li的加入有利于实现更集中地放热。Al-37Mg-3Eu的反应时间也比Al-40Mg的反应时间缩短,其反应温升相比于Al-40Mg也有了很大的提升,Eu的加入也促进了水反应的进行。
为了计算Al、Al-40Mg、Al-37Mg-3Li、Al-37Mg-3Eu粉末与H2O反应的产氢量,水反应结束后将反应釜停止加热并冷却至室温,记录室温下反应釜的压强数据,并通过范德瓦尔斯方程计算产氢量和产氢效率。四种粉末的产氢量和产氢效率汇总在表2中。由表2可知,Al粉的产氢效率为80.17%,而三种合金粉末的产氢效率都超过了90%。值得一提的是,Al-37Mg-3Li和Al-37Mg-3Eu这两种三元合金粉末的产氢效率比Al-40Mg二元合金粉末的产氢效率更高。

Table 2. Hydrogen production and hydrogen production efficiency of Al, Al-40Mg, Al-37Mg-3Li and Al-37Mg-3Eu powders reacting with water
表2. Al、Al-40Mg、Al-37Mg-3Li、Al-37Mg-3Eu粉末与水反应的产氢量和产氢效率
3.3. Al基合金粉末的水反应产物
图7是Al、Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu四种粉末水反应产物的XRD图谱。由图可知,Al粉与H2O的反应不完全,反应产物由AlOOH和残余Al组成。而Al-40Mg、Al-37Mg-3Li、Al-37Mg-3Eu与H2O的反应都比较完全:Al-40Mg与H2O反应的产物为AlOOH和Mg(OH)2,没有残余的Al、Mg或Al-Mg中间相化合物;Al-37Mg-3Li与H2O反应后得到AlOOH、Mg(OH)2和LiAl2(OH)7,反应完全;Al-37Mg-3Eu的水反应产物为AlOOH和Mg(OH)2,Al3Mg2和Al12Mg17已经与H2O反应完全,在产物中没有检测到Eu元素,可能是因为反应产生的Eu(OH)3单独存在,Eu(OH)3太少而没有被检测到。结果表明,在Al中添加Mg形成Al-Mg合金和Al-Mg基合金后,合金粉末与水反应的反应进行得更彻底,反应完成程度更高。


Figure 7. XRD patterns of the reaction products of four powders with water: (a) Al, (b) Al-40Mg, (c) Al-37Mg-3Li, (d) Al-37Mg-3Eu
图7. 四种粉末水反应产物的XRD图谱:(a) Al、(b) Al-40Mg、(c) Al-37Mg-3Li、(d) Al-37Mg-3Eu
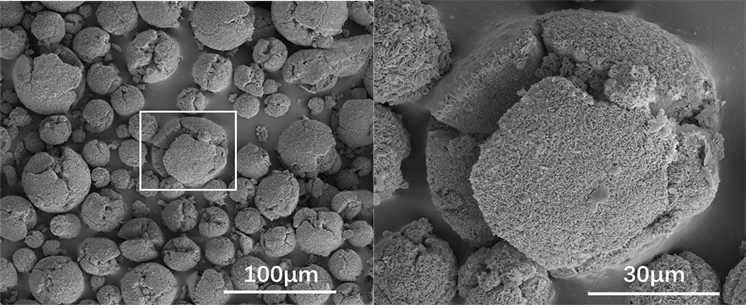
Figure 8. SEM images of reaction product of Al-40Mg with water
图8. Al-40Mg水反应产物的SEM照片

Figure 9. SEM images of reaction product of Al-37Mg-3Eu with water
图9. Al-37Mg-3Eu水反应产物的SEM照片

Figure 10. SEM images of reaction product of Al-37Mg-3Li with water
图10. Al-37Mg-3Li水反应产物的SEM照片
为了进一步分析高镁含量的铝基合金粉末与水反应的过程和机理,对水反应产物进行SEM表征。图8是Al-40Mg与H2O反应结束后产物的SEM图,球形颗粒破裂,表面产生了大而深的裂纹,且表面有大量米粒状的纳米结构。图9是Al-37Mg-3Eu水反应产物的SEM照片,颗粒表面都出现了极深的裂纹,且部分颗粒内部产生了空洞,部分颗粒的球形结构只剩下残留的一小半,还能观察到一些小碎屑。图10是Al-37Mg-3Li水反应产物的SEM照片。图10显示,产物颗粒表面出现了米粒状结构和薄片状结构,并且存在孔洞和深裂纹,其颗粒破碎的程度比Al-40Mg破裂的程度更深。通过对比可知,Al-40Mg、Al-37Mg-3Li、Al-37Mg-3Eu合金粉末水反应产物破碎程度递增,亦表明其水反应完全程度增加。
4. 结论
1. 使用高温气雾化法分别制备了Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu三种合金粉末。三种合金粉末均由Al3Mg2和Al12Mg17两种物相组成,粉末颗粒具有良好的球形度,表面光滑,无明显团聚。
2. 水反应实验、水反应产物的表征结果均表明,Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu合金粉末的水反应性能均优于Al粉。Li能够降低合金粉末与水的反应启动温度,使放热更集中,Eu也能够促进水反应的进行。Al粉的产氢效率为80.17%,Al-40Mg、Al-37Mg-3Li与Al-37Mg-3Eu合金粉末的产氢效率分别提升至93.69%、94.32%与94.63%。
致谢
感谢华中科技大学分析测试中心的指导和帮助。
基金项目
国家自然科学基金资助(51871106)。