摘要: 目的:系统评价伏诺拉生(VPZ)疗法相对于质子泵抑制剂(PPI)疗法在幽门螺杆菌(Hp)感染患者补救治疗中的有效性及安全性。方法:计算机检索The Cochrane Library、Web of Science、PubMed、中国知网、CBM等,检索时限为建库起至2022年5月。如果评估了基于VPZ和基于PPI的疗法之间的根除率,则纳入这些研究,提取相关资料并采用Cochrane系统评价手册推荐的偏倚风险评估工具对随机对照试验进行质量评价,采用纽卡斯尔–渥太华量表对非随机对照试验进行质量评价,使用RevMan 5.3软件进行Meta分析。结果:共纳入7项研究,合计3013例患者。在意向治疗(ITT)分析和符合方案(PP)分析中,VPZ组患者的Hp总体根除率比PPI组显著升高,分别为87.55% vs. 79.55% [RR = 1.07, 95%CI (1.00, 1.13), P = 0.02]和94.07% vs. 87.34% [RR = 1.08, 95%CI (1.04, 1.12), P < 0.0001]。VPZ组患者的不良事件发生率和PPI组不良事件发生率差异无统计学意义,为10.98% vs. 13.03% [RR = 0.65, 95%CI (0.22, 1.95), P = 0.45]。结论:与PPI疗法相比,VPZ疗法在Hp感染患者补救根除治疗中效果更优。VPZ疗法的安全性良好,甚至优于PPI疗法。
Abstract:
Objective: To systematically evaluate the efficacy and safety of vonoprazan (VPZ) therapy relative to proton pump inhibitor (PPI) therapy in the remedial treatment of patients with Helicobacter pylori (Hp) infection. Methods: Computerised searches of The Cochrane Library, Web of Science, PubMed, CNKI, CBM, etc. were performed within a timeframe from the time of library construction to May 2022. Studies were included if eradication rates between VPZ-based and PPI-based therapies were assessed, relevant information was extracted and quality evaluated using the risk of bias assess-ment tool recommended by the Cochrane Handbook of Systematic Evaluation for randomised con-trolled trials, and the Newcastle-Ottawa scale for non-randomised controlled trials, and Me-ta-analyses were performed using RevMan 5.3 software. Results: Seven studies with a combined to-tal of 3013 patients were included. In the intention-to-treat (ITT) analysis and protocol-conformant (PP) analysis, patients in the VPZ group had significantly higher overall Hp eradication rates than those in the PPI group, 87.55% vs. 79.55% [RR = 1.07, 95%CI (1.00, 1.13), P = 0.02] and 94.07% vs. 87.34% [RR = 1.08, 95%CI (1.04, 1.12), P < 0.0001]. The difference between the rate of adverse events in patients in the VPZ group and the rate of adverse events in the PPI group was not statisti-cally significant at 10.98% vs. 13.03% [RR = 0.65, 95%CI (0.22, 1.95), P = 0.45]. Conclusion: VPZ therapy is more effective in remedial eradication therapy in patients with Hp infection compared to PPI therapy. The safety profile of VPZ therapy is favourable and even superior to that of PPI therapy.
1. 引言
幽门螺杆菌(Helicobacter pylori, Hp)是革兰氏阴性菌,呈螺旋形,作为一种广泛的机会致病菌,大约感染全球50%的人口 [1] ,可导致严重的胃和十二指肠疾病,包括消化性溃疡、胃癌、胃黏膜相关组织淋巴瘤等 [2] 。相关的研究表明约90%胃癌与Hp的感染有关 [3] ,世界卫生组织也将其列为致癌物。值得注意的是,相关研究表明根除Hp不仅可以降低胃癌的发生(OR = 0.46, 95%CI: 0.39~0.55) [4] [5] ,还可将早期胃癌患者中转移性胃癌的发生率降低50% (范围为20%~70%) [6] [7] 。因此,成功根除Hp是预防胃癌的重要策略之一。
目前的指南建议所有Hp感染的患者均应进行根除治疗,包含抑酸剂与抗生素的三联或四联疗法作为治疗策略 [2] [8] ,若初次治疗失败则导致Hp感染者对方案中使用的几种抗生素耐药性增加,并使进一步的治疗更加复杂和昂贵 [9] [10] ,因此我国最新指南也鼓励在补救治疗中进行药敏试验以进一步提高根除率 [2] 。
VPZ,作为一种新型的钾离子竞争性酸阻滞剂,与传统的PPI相比,具有起效快,半衰期长,主要经CYP3A4代谢,CYP2C19基因多态性对其影响很小等特点 [11] ,被广泛应用于酸相关疾病。2020年Satoshi Shinozaki表明基于VPZ的方案在二线治疗中优于基于PPI的方案 [12] ,最新的一项回顾性临床研究得出,含VPZ方案在Hp感染的补救治疗中是有效且安全的 [13] ,但患者数量和研究不足以适当评估两种方案之间根除成功率的差异。本研究旨在阐明基于VPZ的方案与基于PPI的方案相比作为补救治疗根除Hp感染的有效性和安全性。
2. 方法
2.1. 检索策略
检索The Cochrane Library、Web of science、PubMed英文数据库和中国知网、维普和万方数据、CBM等中文数据库,收集相关临床研究,检索时限为各数据库建库起至 2022年5月。采用以下检索词:英文检索词包括:“vonoprazan”或“potassium-competitive acid blocker”或“Takecab”(商品名)或“TAK438”(研究代码)或“P-CAB”和“Helicobacter pylori”或“Hp”或“H. pylori”;中文检索词包括:“钾离子竞争性酸阻滞剂”或“新型抑酸药物”或“新型钾离子竞争性酸阻滞剂”或“幽门螺旋菌”或“幽门螺旋杆菌”或“螺旋菌”。采用主题词和自由词结合构成检索策略。同时追溯相关研究的参考文献。
2.2. 研究选择
首先,我们通过阅读标题和摘要来排除与研究内容不相关的文章。其次,我们根据预先设计的纳入和排除标准选择临床研究。纳入标准如下:(1) 患者:已确诊Hp感染,非初次治疗;(2) 干预:基于VPZ方案的根除治疗,包括三联治疗、二联治疗;(3) 对照:基于PPI方案的根除治疗,包括三联疗法,铋剂四联疗法;(4) 结局:Hp的根除率和不良事件发生率;(5) 研究设计:随机对照研究(RCT)和非随机对照研究(NRCT)。排除标准如下:(1) 健康人群或小于18岁的人群;(2) 无法获得的全文;(3) 研究类型为综述、信件、会议报告等;(4) 无满足要求的结局变量指标或原始数据。两名调查人员独立评估该研究的资格,出现任何分歧请第三位研究者协助判断。
2.3. 研究终点
本文的研究指标包括:(1) Hp根除率(主要指标);(2) 不良事件发生率(次要指标)。
2.4. 数据提取
提取以下资料:第一作者、发表日期、研究对象的性别和年龄、研究持续时间、样本量、治疗天数、药物剂量、根除次数、根除率、不良事件。
2.5. 文献质量的评估
RCT采用Cochrane手册推荐的偏倚风险评价工具 [14] 进行文献质量评价,包括6个方面:(1) 随机分配方法;(2) 分配隐藏;(3) 是否采用盲法;(4) 结果数据的完整性;(5) 选择性报告结果;(6) 其他偏倚来源。对每项内容作出“低风险”“高风险”“不确定”的判断。NRCT采用纽卡斯尔–渥太华量表(Newcastle-Ottawa Scale, NOS) [15] 进行文献质量评价,包括3个方面:(1) 研究对象的选择;(2) 研究组间的可比性;(3) 结果测量。NOS总分为9分,总分 > 5分则认为文献的方法学质量较高。
2.6. 统计学方法
本系统评价采用Revman软件(5.3.1版)进行。采用Cochranes’ Q检验和I2统计量来检测异质性,当P > 0.1且I2 < 50%时,各研究间存在统计学异质性,使用随机效应模型进行分析,反之则使用固定效应模型。采用风险比(RR)和相应的95%置信区间(CI)来确定基于VPZ的方案和基于PPI的方案的有效性及安全性。P ≤ 0.05认为差异有统计学意义。
3. 结果
3.1. 文献筛选结果及基本特征
通过检索共获得568篇文献,初步剔除重复文献和系统评价后剩余375篇,通过阅读题目和摘要后不符合的有266篇,纳入109篇文献,进行阅读全文后剔除102篇,最终纳入7篇文献。共纳入3013例患者,其中干预组940例患者,对照组2073例患者。纳入文献的基本特征见表1。

Table 1. Basic characteristics of the included studies
表1. 纳入研究的基本特征
V:伏诺拉生;A:阿莫西林;C:克拉霉素;L:兰索拉唑;E:埃索美拉唑;R:雷贝拉唑;B:铋剂;F:呋喃唑酮;M:甲硝唑;S:西他沙星;①:根除率;②:不良事件。
3.2. 文献质量的评价
纳入的1篇RCT [16] 研究中,报道了随机序列的产生过程,评价为“低风险”;未报道分配方法是否隐藏,评价为“不确定”,对研究对象和人员均未采用盲法,评价为“高风险”;在随访过程中均有失访人员且报道了失访原因,评价为“低风险”;无选择性报告结果,评价为“低风险”,此研究的偏倚风险较低。
NRCT [17] - [22] 的质量评价见表2,总分 > 6分,则认为方法学质量较高。

Table 2. Analysis of bias in NRCTs
表2. 非随机对照研究的偏倚分析
3.3. Meta分析的结果
3.3.1. Hp根除率的比较
(1) 总体比较:
1. 在ITT分析中,纳入研究 [16] - [22] 存在统计学异质性(I2 = 57%, P = 0.03),选择随机效应进行meta分析,结果显示基于VPZ的方案优于PPI方案,RR = 1.07 (1.01, 1.13)且具有统计学意义(Z = 2.24, P = 0.02),详见图1。
2. 在PP分析中,纳入研究 [16] - [22] 存在统计学异质性(I2 = 45%, P = 0.09),故采用随机效应模型进行meta分析,结果显示基于VPZ的方案优于PPI方案,RR = 1.08 (1.04, 1.12),且具有统计学意义(Z = 4.03, P < 0.0001),详见图2。
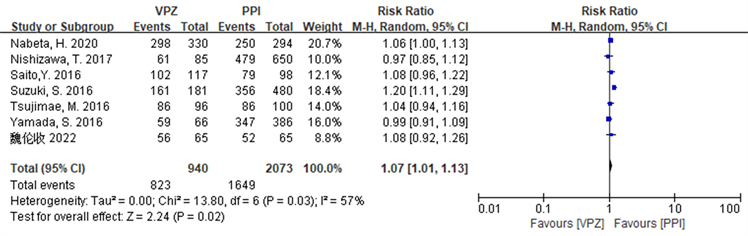
Figure 1.Forest plot of ITT analysis comparing the overall Hp eradication rate in the two groups
图1. 两组患者Hp总体根除率比较的ITT分析森林图

Figure 2. Forest plot of PP analysis comparing the overall Hp eradication rate in the two groups of patients
图2. 两组患者Hp总体根除率比较的PP分析森林图
3. 敏感性分析
Hp总体根除率的敏感性分析结果显示,在ITT分析,剔除文献 [19] [21] 后,各研究的异质性较前下降,Q检验的P = 0.03升至0.75,I2由57%降至0%,Meta分析结果显示,干预组患者的Hp总体根除率仍高于对照组(P < 0.05),与剔除前一致,详见图3。在PP分析中,剔除文献 [20] ,Q检验的P = 0.09升至0.11,I2 = 45%不变,Meta分析结果显示,干预组患者的Hp总体根除率仍优于对照组(P < 0.05),与剔除前一致,详见图4。
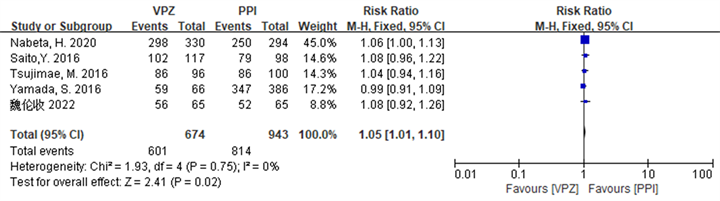
Figure 3. Forest plot of ITT analysis comparing the overall Hp eradication rates of the two groups of patients after sensitivity analysis
图3. 敏感性分析后两组患者Hp总体根除率比较的ITT分析森林图
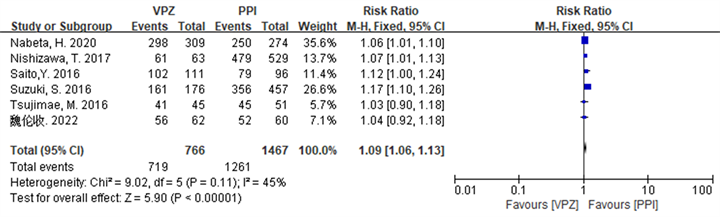
Figure 4. Forest plot of PP analysis comparing the overall Hp eradication rates of the two groups of patients after sensitivity analysis
图4. 敏感性分析后两组患者Hp总体根除率比较的PP分析森林图
(2) 亚组分析
1. 在二线治疗亚组中Hp根除率分析
在ITT分析中,纳入研究 [16] [17] [18] [19] [20] [22] 无统计学异质性(I2 = 4%, P = 0.39),选择固定效应模型进行meta分析,结果显示基于VPZ的方案优于PPI方案,RR = 1.02 (0.98, 1.07),但不具有统计学意义(Z = 1.07, P = 0.28 > 0.05),详见图5。在PP分析中,纳入研究 [16] [17] [18] [19] [20] [22] 无统计学异质性(I2 = 0%, P = 0.93),故采用固定效应模型进行meta分析,结果显示基于VPZ的方案优于PPI方案,RR = 1.05 (1.02~1.08),且具有统计学意义(Z = 3.32, P = 0.0009 < 0.05),详见图6。

Figure 5. Forest plot of ITT analysis comparing Hp eradication rates in the two groups in the second-line treatment subgroup
图5. 两组Hp根除率在二线治疗亚组中比较的ITT分析森林图
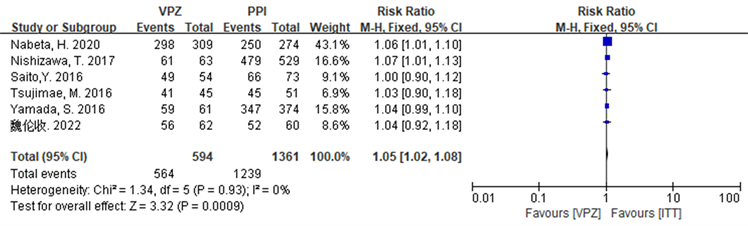
Figure 6. Forest plot of PP analysis comparing Hp eradication rates in the two groups in the second-line treatment subgroup
图6. 两组Hp根除率在二线治疗亚组中比较的PP分析森林图
2. 在试验地为日本亚组中Hp根除率分析
在ITT分析中,纳入研究 [17] - [22] 存在统计学异质性(I2 = 64%, P = 0.02),选择随机效应模型进行meta分析,结果显示基于VPZ的方案优于PPI方案,RR = 1.06 (1.00~1.13),具有统计学意义(Z = 1.98, P = 0.05),详见图7。在PP分析中,纳入研究 [17] - [22] 存在统计学异质性(I2 = 54%, P = 0.05),故采用随机效应模型进行meta分析,结果显示基于VPZ的方案优于PPI方案,RR = 1.08 (1.04~1.12),且具有统计学意义(Z = 3.77, P = 0.0002),详见图8。
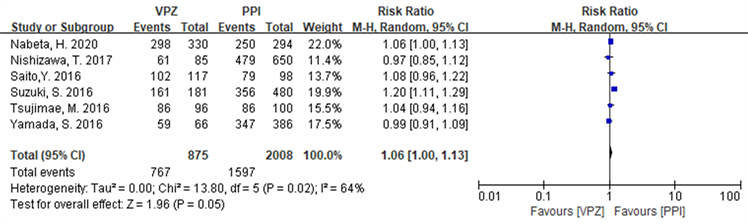
Figure 7. Forest plot of ITT analysis comparing Hp eradication rates in the two groups in the Japanese subgroup
图7. 两组Hp根除率在日本亚组中比较ITT分析森林图
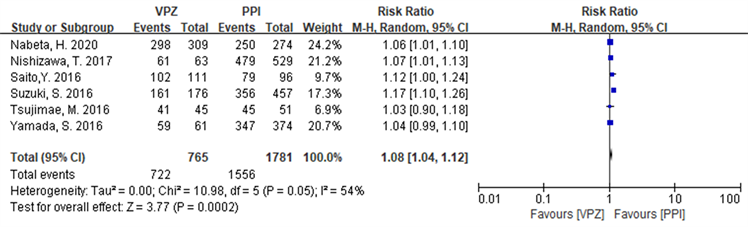
Figure 8. Forest plot of PP analysis comparing Hp eradication rates in the two groups in the Japanese subgroup
图8. 两组Hp根除率在日本亚组中比较PP分析森林图
3.3.2. 不良事件发生率的比较
2篇文献 [16] [21] 描述了不良事件的发生,且发生率存在统计学异质性(I2 = 72%, P = 0.06),故选择随机效应模型进行meta分析,结果显示基于VPZ的方案安全于PPI方案,RR = 0.65 (0.22~1.95),但不具有统计学意义(Z = 0.78, P = 0.45),详见图9。
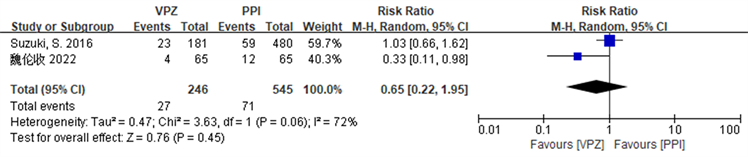
Figure 9. Forest plot comparing the incidence of adverse events in the two groups of patients
图9. 两组患者不良事件发生率比较的森林图
4. 讨论
Hp影响着世界范围内数十亿人,尽管全世界有30年的治疗经验,但理想的根除方案仍不清楚。目前,临床批准PPI和2种抗生素,可联合或不联合铋剂作为根除Hp的治疗方案 [2] [8] [10] 。而随着部分抗生素耐药率的增加以及酸抑制不足等问题 [23] ,传统的含PPI的疗法相关根除率持续下降已成为全球关注的问题 [24] [25] ,其中铋剂四联疗法根除率为81.3%,PPI和2种抗生素的根除率为75.7% [26] 。因此提出了许多措施来改善Hp的根除策略,包括使用较高剂量或较强的抑酸药物提高胃酸pH值 [11] [25] [27] 。研究表明,当胃内pH为6~8.31时,Hp处于生长状态,且对抗生素敏感,易于被清除 [28] 。而VPZ,作为一种新型的抑酸剂,由于其起效快,具有强大而持久的抑酸作用已被推荐用于Hp的治疗 [10] [29] 。
本研究整体根除率的ITT和PP显示,含有VPZ的方案均高于含PPI的方案,ITT中含VPZ方案与含PPI方案的根除率分别为87.55% vs 79.55% (P < 0.05),PP分析中分别为94.07% vs 87.34% (P < 0.05),与既往一项真实性回顾性研究结果类似,均证明了VPZ在补救治疗中的优越性 [13] ,这可能与VPZ具有更强的抑酸效果和更长时间维持胃内pH > 6有关。VPZ作为一种可逆的竞争性酸阻滞剂 [30] ,通过与H+/K+-ATP酶共价结合后,能在4小时内使得胃内pH > 7 [31] ,显示出PPI更强的不依赖酸活性的抑酸作用 [32] [33] 。二线治疗的亚组分析中,ITT分析结果显示含VPZ的方案较PPI方案其根除率更高,差异无统计学意义(86.75% vs. 81.58%, P = 0.28),与既往Dong,S.Q等人得出的结果类似 [34] ;PP分析结果与ITT类似,但是其差异有统计学意义(94.95% vs. 91.04%, P = 0.0009),这一结果与Shinozaki,S等人分析的结果类似 [12] ,造成这一差异的主要原因可能是:本文纳入的文献数量有限,样本量较小,限制了证据水平,另外,因本亚组分析中仅包含1项RCT,可能导致结果出现偏倚,尚需更多的研究来验证这一结论。在日本研究亚组中,无论在ITT分析还是PP分析中,含VPZ的治疗方案均较PPI方案显示出显著优势(ITT: 87.66% vs. 79.53%, P = 0.05; PP: 94.38% vs. 87.37%, P = 0.0002),与魏伦收 [16] 在中国得出的结果存在差异(ITT: 86.15% vs. 80.00%, P = 0.35; PP: 90.32% vs. 86.67%, P = 0.53),原因可能是:幽门螺杆菌根除方案的成功取决于人群中的耐药模式和人群中药物代谢酶的常见宿主基因型,以及幽门螺杆菌对常用抗菌药物耐药的流行率因地区而异,并且与不同地区的抗生素消费有关 [35] ,此外我们仅与1篇中国RCT进行了比较,随着样本量的增加,结果可能会发生变化。
本研究中,总体根除率的ITT分析和PP分析显示各研究间存在较为显著的异质性,对此,进行了敏感性分析,剔除相应文献后,干预组与对照组之间异质性明显降低,且根除率仍存在统计学意义,即在Hp补救治疗中VPZ方案的根除率优于PPI方案,提示本研究结论稳健、可靠。
在本研究中,我们还评估了VPZ和PPI作为Hp根除治疗的安全性。含VPZ方案的不良事件发生率虽低于含PPI方案,但是两组间不良事件发生率差异无统计学意义(10.98% vs. 13.03%, P = 0.45)。因此,含VPZ的Hp根除治疗的安全性和耐受性是可以接受的。
虽然本系统评价显示了基于VPZ方案在Hp补救治疗中的优势,但也存在如下局限性:首先,纳入研究中只有1篇是在中国进行的RCT研究,其余都是在日本进行的NRCT研究,由于抗生素耐药性因国家而异,且缺乏来自欧洲和美国国家的数据可能造成了选择性偏差,因此有必要在多个国家进行临床试验以验证本研究的结论。其次,用于分析VPZ和PPI作为补救治疗差异的研究数量和样本量有限。第三,没有评估抗生素耐药性的作用。第四,各研究采用了不同的抗生素进行补救治疗。最后,我们只比较了7天VPZ三联方案的疗效,没有考虑其他根除治疗的方案。
5. 结论
综上所述,对于需要补救治疗的患者而言,与PPI疗法比较,含VPZ的治疗方案效果更优,且其安全性良好,甚至优于PPI疗法。本meta分析的结果证据支持VPZ应用于Hp补救根除的治疗实践,具有一定临床应用价值。
NOTES
*通讯作者。