1. 引言
氟污染作为构成人类生存环境的主要威胁之一,除个别地区是因自然因素,大量的高含有氟的工业废水的排放是其产生的主要因素。我国含氟废水排放源主要包括三类:应用氟化物为原料加工生产其它产品的工业;以莹石为原料直接生产氟化物的工业;因工业原料中含氟而生产中排放出氟的工业。由于国内部分生产厂的处理设施不是很完善,所以排放的废水中氟含量超过国家的排放标准,严重污染了环境。国家污水综合排放标准规定,废水中氟离子质量浓度应小于10 mg/L;饮用水中氟离子质量浓度小于1 mg/L[1] 。水滑石为阳离子胶体微粒,有较大的比表面积、良好的水分散性及层间阴离子可交换性,常作为吸附剂用于水处理。鉴于其具有结构记忆效应,焙烧后的复合氧化物也可用作阴离子吸收剂[2] 。壳聚糖是自然界广泛存在的几丁质经过脱乙酰作用得到的,化学名称为聚葡萄糖胺(1, 4)-2氨基-B-D葡萄糖,该天然的高分子被广泛应用在各个领域,并在食品,医学,化工,水处理,生物医学工程等诸多领域研究中取得重大进展[3] -[5]。在特定条件下,壳聚糖能发生水解、酰基化、烷基化、磺化、硝化、羧甲基化、卤化、氧化还原、缩合、络合等反应,可以得到各种不同性质的壳聚糖衍生物。壳聚糖分子中含有大量的羟基和氨基,他们具有较强的化学成键能力。在碱性条件下C-6上的羟基可以发生羟乙基化、羧甲基化、磺酸酯化等,反应中大量引入了侧基,提高其溶解度。壳聚糖具有显著的吸附能力,许多金属离子都可以被壳聚糖吸附,如:镁,钾,锌,钙,铀。壳聚糖的吸附活性能够有选择的发挥,脱乙酰度越大,吸附能力越强[6] 。基于壳聚糖的优势,本文选用壳聚糖为模板制备壳聚糖基NiAl-LDHs,使得NiAl-LDHs的层板可弯曲,并对其饮水除氟性能进行了研究。
2. 实验部分
2.1. 仪器与药品
HT7700-透射电镜,X-RAY DIFFRACTOMETER(日本理学Rigaku公司),XH-MC-1微波合成仪,DTG6-0 热重分析仪,Nicolet is10傅立叶变换红外光谱仪,pF-1型氟离子选择电极(上海精密科学仪器有限公司),pHS-3C pH计(上海精密科学仪器有限公司)等。
NaF, Al2(SO4)3·18H2O, Ce(NO3)3·6H2O, γ-Al2O3, NaNO3, C6H5Na3O7·2H2O, NaOH, HCl等试剂均为分析纯。
2.2. 吸附剂的制备方法
方法一:将硝酸镍和硝酸铝以2:1的摩尔比溶于200 mL蒸馏水中,加入0.5~1.0 g壳聚糖。取25%的氨水30~50 mL,用去离子水稀释到200 mL;80℃水浴下缓慢同时滴加两种溶液形成沉淀,并剧烈搅拌,保持溶液的pH值在10左右;滴加完毕后继续搅拌1 h,转移到90℃水浴中晶化24 h;过滤,用去离子水洗至中性,在80℃下烘干,细致研磨即得到样品,记为NiAlCC-LDHs。
方法二:按照Ni/Al的摩尔比为2:1,尿素/金属离子的摩尔比为3:1称量药品。称量8.724 g硝酸镍,5.627 g硝酸铝,加入0.5 g壳聚糖,溶于50 mL去离子水中,超声溶解10 min,转移到90℃水浴锅中搅拌30 min,直到壳聚糖完全溶解。称量8.108 g尿素,溶于50 mL去离子水中。把上述两种溶液混合加入圆底烧瓶中,在N2保护下,微波搅拌反应2 h,微波温度100℃,功率300 W。冷却到室温后抽滤,洗涤,80℃下烘干,细致研磨即得到样品,记为NiAlMC-LDHs。
2.3. 表征
用X射线衍射分析仪,红外光谱仪、透射电镜和热重差热分析对吸附剂进行表征。
2.4. 吸附试验
通过吸附剂用量、焙烧温度、pH值、干扰离子、吸附温度、吸附时间等因素对吸附材料的性能进行考察,找到最优的吸附条件。取0.2 g吸附剂到50 mL广口瓶中,加入50 mL氟离子溶液(10 mg/L),静态吸附24 h。取吸附24 h后的溶液10 mL加入到50 mL容量瓶中,同时加入10 mL总离子强度调节缓冲溶液,用水稀释至标线,摇匀,上述容量瓶中的溶液倒入100 mL小烧杯中,待电位稳定后,测定其电极电势,读取电位值,进而测定其吸附量、去除率等参数。在每次测量之前,都要用去离子水充分洗涤电极,并用滤纸吸去水分。根据测得的毫伏数,由标准曲线得出氟离子溶液的浓度。通过吸附前后溶液中氟离子浓度的变化来计算吸附量,计算公式如下:
 (1)
(1)
其中:qe——吸附量(mg/g);C0——吸附前溶液氟离子的浓度(mg/L);Ce——吸附后溶液氟离子的浓度(mg/L);m——投加吸附剂的质量(mg);V——溶液的体积(L)。
3. 结果与讨论
3.1. 材料表征
3.1.1. XRD
对两种方法制备的NiAlCC-LDHs和NiAlMC-LDHs进行XRD扫描分析,结果如图1所示。微波方法制得的NiAlMC-LDHs衍射峰型尖锐对称,基线平稳,对比NiAlCC –LDHs,具有较好的结晶度。NiAlMC-LDHs在2θ为11.12˚,22.42˚,34.54˚,38.86˚,60.48˚和61.50˚分别符合水滑石的(003),(006),(012),(015),(110)和(113)特征峰,共沉淀方法制备的NiAlCC-LDHs的(110)和(113)衍射峰堆叠在一起。制备的壳聚糖基NiAl-LDHs与传统MgAl水滑石(分子式Mg6Al2(CO3)(OH)16·4H2O)相似,衍射峰位置向右偏移,这是由于Mg2+和Ni2+的半径差异所致,Mg2+的离子半径0.72 Å,Ni2+的离子半径为0.69 Å。晶体参数见表1所示。晶胞参数a0等于层板上相邻阳离子之间的距离,该值与构成水滑石层板的金属阳离子半径大小有关[7] 。根据(110)衍射峰的位置,计算a0值,a0 = 2 d (110)。水滑石的层间距c0等于单层层板厚度加上层间距离,由图1可知,样品为3R多形体,c0 = c/3,通过低角度(003)衍射峰计算。由表1
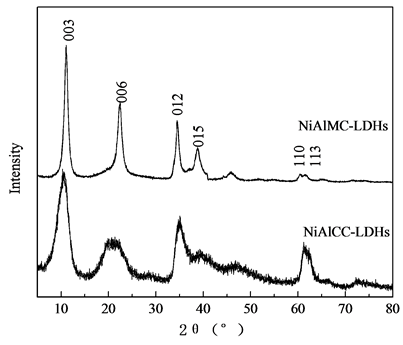
Figure 1. XRD patterns of samples
图1. 样品的XRD谱图

Table 1. Structure parameters of samples
表1. 材料的结构参数
可知,共沉淀方法制得的样品层间距c0 = 8.292 Å,大于微波方法制得的样品,大的层间距有利离子交换,更利于吸附。
根据Scherrer公式计算晶粒尺寸,利用(110) 和(003)的半峰宽。Scherrer公式:
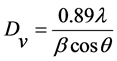 (2)
(2)
其中:λ为CuKα波长 (λ = 1.54056 Å),β为半高宽强度,θ为对应的晶面衍射角。
3.1.2. TG-DTA
对壳聚糖,不添加壳聚糖NiAl-LDHs,NiAlCC-LDHs和NiAlMC-LDHs四个样品进行热重分析,结果如图2。图2(A)天然壳聚糖失重分两个阶段,分别在250℃和562℃下。DTA曲线在70.7℃处有一个吸热峰,在该温度下,壳聚糖失去一些表面吸附水。在172℃下,壳聚糖中一些小的键断裂吸热,有趣的是,当温度升高到300℃~500℃之间,DTA曲线有一个平台,在该温度下壳聚糖主链断裂,一般是先断裂醚键,接着C-O,C-H键断裂。当温度达到562℃时,壳聚糖完全分解,最终裂解成二氧化碳,水,氮氧化物等。壳聚糖中含有氨基,在酸性介质中H+与壳聚糖分子链上的-NH2作用,形成-NH3+阳离子,即质子化作用,使得壳聚糖链上带有大量正电荷,成为阳离子聚电解质,从而对水中阴离子污染物具有强的吸附效应[8] 。高温焙烧后使得壳聚糖链分解破坏,对F-的吸附性能反而减弱。
图2(B)为不添加壳聚糖的NiAl-LDHs热分解图,NiAl-LDHs的失重分两个阶段:当温度在30℃~230℃变化,首次质量损失约为13.8%,该温度下主要失去物理吸附的水合层间水分子,对应DTA曲线上在187℃作用有一个向下的吸热峰。升高温度到800℃,该阶段质量损失约为24.24%,在DTA曲线上335℃处有一个明显的向下吸热峰,该阶段NiAl-LDHs主要脱去层板-OH和层间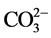 ,
,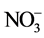 分解,主要分解产物CO2和NO,层间阴离子分解,最终导致水滑石层板破坏,形成尖晶石结构物质[9] 。图2(C)和2(D)分别为壳聚糖复合NiAlCC-LDHs和NiAlMC-LDHs的热分解图。添加壳聚糖的NiAl-LDHs其热分解性能大大改变,分解主要分两个阶段,温度在170℃左右水滑石主要失去表面物理吸附的水分子,对应DTA曲线相有向下的吸热峰;当温度达到300~385℃,DTA曲线上有向上的放热峰,对比壳聚糖的热分解图,该分解温度与壳聚糖分解温度相符,可知,在该温度下NiAlCC-LDHs和NiAlMC-LDHs中复合的壳聚糖发生分解,放出热量,说明壳聚糖与水滑石很好的复合在一起。并且在340℃左右伴随水滑石脱去层板-OH和层间阴离子[10] 。当440℃左右处水滑石的层板完全坍塌,形成尖晶石结构,该温度明显小于文献中报道的水滑石的最终分解温度,这可能是由于壳聚糖的添加,壳聚糖粘性使得水滑石的层板弯曲[11] ,在该温度下壳聚糖主链断裂,使得水滑石的层板破裂,导致其结构的破坏。
分解,主要分解产物CO2和NO,层间阴离子分解,最终导致水滑石层板破坏,形成尖晶石结构物质[9] 。图2(C)和2(D)分别为壳聚糖复合NiAlCC-LDHs和NiAlMC-LDHs的热分解图。添加壳聚糖的NiAl-LDHs其热分解性能大大改变,分解主要分两个阶段,温度在170℃左右水滑石主要失去表面物理吸附的水分子,对应DTA曲线相有向下的吸热峰;当温度达到300~385℃,DTA曲线上有向上的放热峰,对比壳聚糖的热分解图,该分解温度与壳聚糖分解温度相符,可知,在该温度下NiAlCC-LDHs和NiAlMC-LDHs中复合的壳聚糖发生分解,放出热量,说明壳聚糖与水滑石很好的复合在一起。并且在340℃左右伴随水滑石脱去层板-OH和层间阴离子[10] 。当440℃左右处水滑石的层板完全坍塌,形成尖晶石结构,该温度明显小于文献中报道的水滑石的最终分解温度,这可能是由于壳聚糖的添加,壳聚糖粘性使得水滑石的层板弯曲[11] ,在该温度下壳聚糖主链断裂,使得水滑石的层板破裂,导致其结构的破坏。
3.1.3. FTIR
为了得到样品的官能团信息,对样品进行了红外谱图的测定。如图3所示:壳聚糖中的氨基和羟基是吸附的主要官能团,壳聚糖在3440 cm−1左右处为O-H,N-H和多糖中氢键的伸缩振动峰。在2929 cm−1和2880 cm−1处为壳聚糖主链的C-H伸缩振动峰和弯曲振动峰,在1653 cm−1和1604 cm−1处的两个特征峰为NHCO中C=O伸缩振动和-NH2中N-H伸缩振动峰[12] 。在1383 cm−1峰为CH3的对称弯曲振动引起。在1158 cm−1处和1086 cm−1处为壳聚糖中C-O的伸缩振动峰。
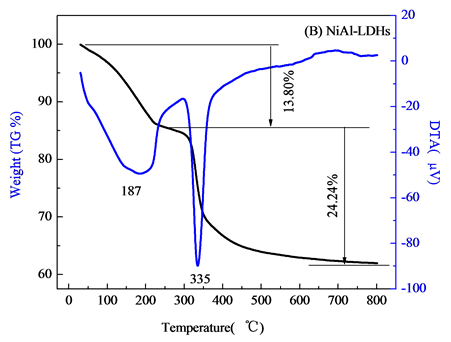
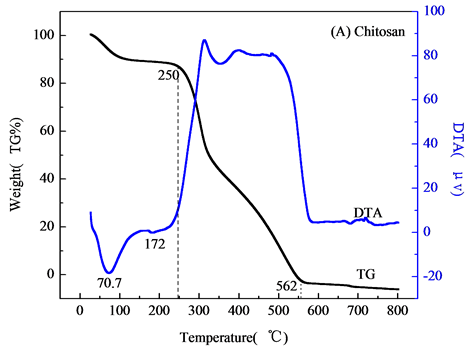
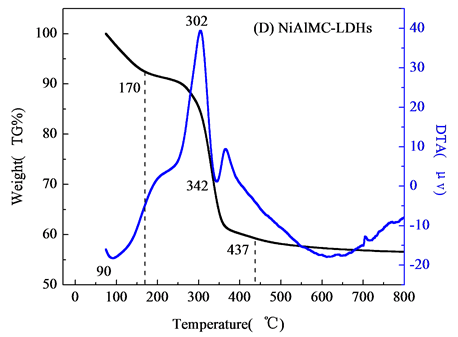
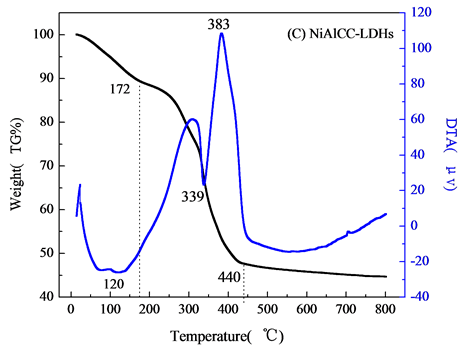
Figure 2. TG-DTA curves of samples (A) Chitosan, (B) NiAl-LDHs; (C) NiAlCC-LDHs; (D) NiAlMC-LDHs
图2. 材料的热重-差热曲线(A) 壳聚糖 (B) NiAl-LDHs (C) NiAlCC-LDHs (D) NiAlMC-LDHs)

Figure 3. The Fourier transform infrared spectra of samples
图3. 样品的红外光谱图
NiAl-LDH在3510 cm−1左右的吸收峰为水滑石表面吸附水分子及层间水分子中-OH的伸缩振动峰,对比添加壳聚糖后的NiAlCC-LDHs和NiAlMC-LDHs该峰向低波数处移动,无氢键的-OH伸缩振动一般在3600 cm−1处,由于壳聚糖中含有大量氢键,所以导致该峰移动。在1635~1647 cm−1处为水分子的角度形变所致,由于层间结构的不同该值略有不同,这与文献中报道相一致[13] 。在1383 cm−1尖锐的吸收峰为层间NO3-离子的振动引起[14] ;在400~800 cm−1为水滑石层板上Ni(Al)-OH,Ni(Al)-O-Ni(Al)和O-Ni(Al)- O的振动引起[15] [16] 。对比NiAlCC-LDHs和NiAlMC-LDHs的红外谱图,微波合成的NiAlMC-LDHs在2184 cm−1处有明显的峰,这是因为微波合成是通过尿素的缓慢水解提供碱环境形成水滑石,而共沉淀方法用氨水作为沉淀剂,该峰是由于尿素水解形成的中间产物OCN−中C≡N三键的振动引起。
3.1.4. TEM
图4分别不同放大倍率下的微波辅助法合成的纯镍铝水滑石和以天然高分子材料壳聚糖为模板的镍铝水滑石透射电子显微镜图,从图3~4中可以看出水滑石样品晶粒均由许多片层结构叠加而成,其结构比较规整,表明其结晶度较好,这与XRD分析结果一致。
比较未掺杂壳聚糖(a和b)和掺杂壳聚糖的镍铝水滑石(c和d)的结构形态,微波辅助法合成的不掺杂壳聚糖的镍铝水滑石的形貌要好于掺杂壳聚糖的水滑石样品。这可能是因为以壳聚糖为模板的镍铝水滑石中壳聚糖的交联作用使合成的样品层板弯曲,从而导致层状结构不如纯镍铝水滑石样品的形貌好。
3.2. 焙烧温度对除氟性能影响
对共沉淀方法和微波方法制备的NiAl-LDHs/壳聚糖进行焙烧处理,焙烧温度(150℃~450℃),焙烧温度对水滑石的除氟性能影响如图5所示,图5(A)为NiAlCC-LDHs,图5(B)为NiAlMC-LDHs对氟离子吸
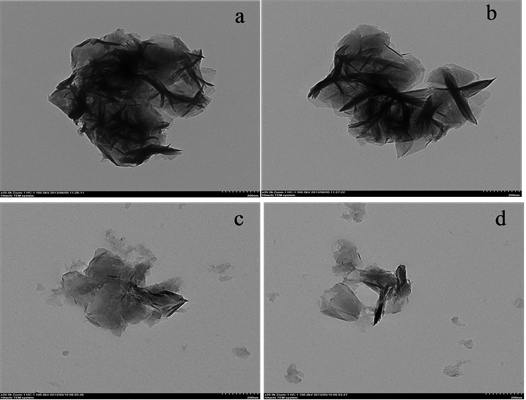
Figure 4. TEM images of samples (a,b: NiAl-LDHs c,d: NiAlMCLDHs)
图4. 微波辅助法制备镍铝水滑石样品的TEM图片(a和b为镍铝水滑石;c和d为壳聚糖基镍铝水滑石)
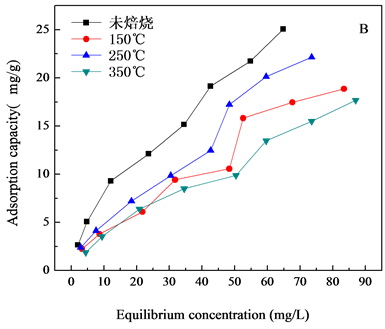
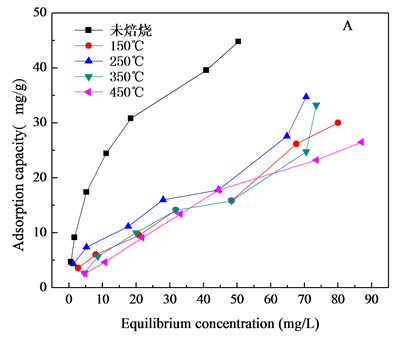
Figure 5. Effect of different calcination temperature on adsorption capacity (A, NiAlCC-LDHs; B NiAlMC-LDHs)
图5. 焙烧温度对吸附量的影响
附性能的影响。焙烧温度从150℃升高到250℃材料的吸附量增大,从250℃到350℃,材料对F−的吸附量降低,焙烧后与未焙烧的材料对比,未焙烧水滑石对F−的吸附性能最好,未焙烧NiAlCC-LDHs和NiAlMC-LDHs对140 mg/L的F−溶液最大吸附量分别为44.80 mg/L和25.05 mg/L。虽然文献中大部分报道焙烧对水滑石的吸附性能有所提高[17] -[19],本研究出现这种结果的原因分析是由于壳聚糖的加入,壳聚糖具有很高的交联性,在172℃左右就会有键的断裂,在300℃开始分解,由于水滑石生长在壳聚糖基底上,壳聚糖的断裂对水滑石层板具有一定的破坏性,层板坍塌,所以焙烧后材料的吸附性能反而没有未焙烧的好,这也可以从TG-DTA曲线(图2)中得到证明。因此未了较好的去除F−离子,选择80℃下烘干未焙烧的材料做吸附剂,研究其对水中F-的去除性能。
3.3. 吸附剂用量对除氟性能影响
吸附剂用量的选取并非越多越好,考虑到吸附剂价格对实际应用的影响,为了使F−与吸附剂位点之间相互作用最大,找到最优用量,对10 mg/L的F−溶液进行了吸附剂用量的研究。NiAlCC-LDHs和NiAlMC-LDHs对F-的去除影响如图6所示:由图可以看出,随着吸附剂用量的加大,吸附量逐渐减小,去除率逐渐增大。当NiAlCC-LDHs和NiAlMC-LDHs的用量分别从0.2 g/L增加到2.0 g/L和4.0 g/L,材料对F−的去除率快速增大,之后接近平衡。这是由于刚开始时,增加吸附剂质量,使得溶液中可用的吸附位点增多,导致了一个快的去除效率。然而,当溶液中的F−几乎全被吸附后,再继续增加吸附剂用量,也不会对F−去除率有贡献[20] 。另外,吸附剂添加过多,可能导致吸附剂的聚集,也会对吸附产生不利影响。两种样品对比,NiAlCC-LDHs(图6(A))具有较高的吸附氟离子性能,当吸附剂用量为2.0 g/L时,NiAlCC-LDHs对F-溶液吸附量分别为4.587 mg/g,吸附后溶液中氟离子浓度仅为0.8241 mg/L,去除率达到91.76%,远远小于世界卫生组织的最低标准1.5 mg/L,达到饮用水标准;当用2.0 g/L NiAlMCLDHs(图6(B))时,去除率仅为74.40%,吸附后溶液中F-浓度2.560 mg/L达不到饮水标准,当用量增加到4.0 g/L时,吸附后溶液中F-浓度1.422 mg/L,同等情况下,NiAlCC-LDHs比NiAlMC-LDHs的吸附性能约高23.33%。综合考虑吸附效果和价格因素,NiAlCC-LDHs和NiAlMC-LDHs的除氟研究分别选择2.0 g/L和4.0 g/L进行试验。
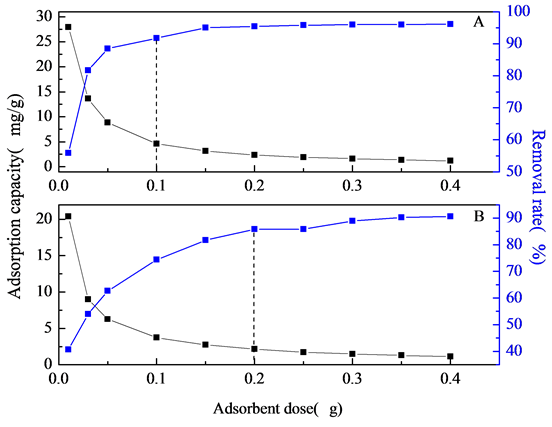
Figure 6. Effect of dosage on the removal of fluoride performance (A, NiAlCC-LDHs; B, NiAlMC-LDHs)
图6. 吸附剂量对氟离子去除性能的影响
3.4. 溶液pH对吸附性能的影响
溶液的pH往往影响材料官能团的质子化作用,为了考察溶液pH值对材料吸附性能的影响,选取10 mg/L的F−溶液,通过稀HCl和NaOH调节pH值在2~12之间变化。不同pH值溶液对材料除氟性能的影响如图7所示:当溶液的pH值在3 < pH < 10之间,材料对氟离子具有较高的去除率,在pH = 7.22时,NiAlCC-LDHs和NiAlMC-LDHs吸附后F-的最大去除率达到91.75%和80.10%。当pH < 3时,去除率分别为51.96%和45.51%,这可能是溶液中H+浓度过大,导致水滑石层板溶解,使其层状结构破坏,对去除氟离子有影响;当pH > 10时,溶液中OH−浓度增大,OH−与F−之间形成竞争作用,同样对氟离子的去除有较大影响,pH = 11.91时,材料对F−的去除率仅为57.64%,47.75%。
pH值对除氟的影响机理推测:
强酸的条件下:
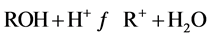 (3)
(3)
酸性使得水滑石的层板溶解,结构被破坏,因此吸附性能降低。
当3 < pH < 10:
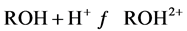 (4)
(4)
材料表面带有部分正电荷,通过静电引力吸附F−,同时F−与材料层间及表面形成氢键,因此适当的pH有利于吸附的进行。另外文献中报道,壳聚糖的吸附受pH值的影响,由于壳聚糖中含有大量的−NH2和−OH,酸性条件下:
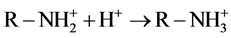 (5)
(5)
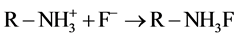
 (6)
(6)
壳聚糖的质子化作用使得其带有正电荷,通过静电引力和形成氢键对水中阴离子有较强的吸附作用,所以偏酸性溶液对吸附有利,最佳吸附pH范围4~7。
在强碱的条件下:
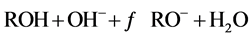 (7)
(7)
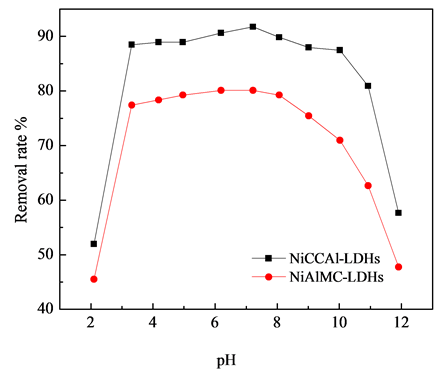
Figure 7. Effect of solution pH on removal rate of fluoride
图7. 溶液pH对氟离子去除率的影响
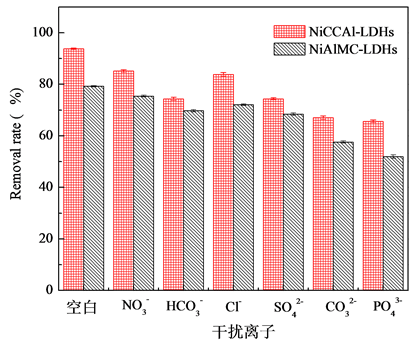
Figure 8. Influence of co-existing interfering ions during the adsorption of fluoride onto NiAlCC-LDHs and NiAlMC-LDHs
图8. 共存离子对吸附氟离子的影响
过度的OH−在材料表面富集,排斥F−,与F−竞争吸附位点,减少F−与材料表面的接触概率,因而F−的去除率降低。强酸和强碱性条件吸附都不好,因此为了比较NiAlCC-LDHs和NiAlMC-LDHs的吸附性能,所有实验pH选择6~7。
3.5. 水中阴离子对除氟性能的影响
实际水体中往往还含有其他阴离子,各种离子的共存对F−的吸附也有影响,为了考察材料对F−的吸附选择性,对水中存在的各种阴离子进行模拟干扰实验,以空白为参照,F−溶液浓度为10 mg/L,各个干扰离子(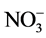 ,
,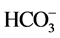 , Cl−,
, Cl−,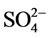 ,
,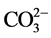 ,
,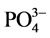 )的浓度为F−浓度的5倍,结果如图8所示。
)的浓度为F−浓度的5倍,结果如图8所示。
阴离子对F−去除率的影响顺序如下: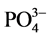 >
> 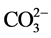 > S
> S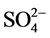 ≈
≈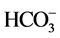 > Cl− >
> Cl− >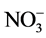 ,阴离子所带电荷越多对F−吸附干扰的作用越大,由于水滑石层板带有正电荷,依靠静电引力与F−结合,阴离子的化合价越高,静电引力越大,与吸附剂结合的越牢固,因而对氟离子的影响也越大。另外,壳聚糖对负电性强的阴离子吸附作用更大,通过氢键将阴离子紧紧吸附在链状结构中。带有一个单位负电荷的
,阴离子所带电荷越多对F−吸附干扰的作用越大,由于水滑石层板带有正电荷,依靠静电引力与F−结合,阴离子的化合价越高,静电引力越大,与吸附剂结合的越牢固,因而对氟离子的影响也越大。另外,壳聚糖对负电性强的阴离子吸附作用更大,通过氢键将阴离子紧紧吸附在链状结构中。带有一个单位负电荷的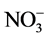 和Cl−对F−的吸附干扰较小,当溶液中存在这两种阴离子时,NiAlCC-LDHs和NiAlMC-LDHs对F−的去除率达到85.17%,75.46%和83.87 %,72.16%。当溶液中存在
和Cl−对F−的吸附干扰较小,当溶液中存在这两种阴离子时,NiAlCC-LDHs和NiAlMC-LDHs对F−的去除率达到85.17%,75.46%和83.87 %,72.16%。当溶液中存在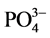 时,两种吸附剂对氟离子的去除率分别为65.66 %和51.96%。
时,两种吸附剂对氟离子的去除率分别为65.66 %和51.96%。
4. 结论
通过传统共沉淀方法和微波辅助方法制备壳聚糖基NiAl-LDHs,壳聚糖的添加有效的改善了水滑石的性能,为水滑石引入了大量的-NH2和-OH官能团,其中-NH2的质子化作用提高了水中F−的去除效,NiAlCC-LDHs的吸附性能高于NiAlMC-LDHs。另外对溶液pH,水中干扰离子影响进行测试,在pH偏酸性条件下材料的吸附性能较高,水中存在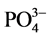 对材料的除氟性能影响最大,是因为阴离子所带电荷越高,与吸附剂表面的静电引力越大,吸附越牢固。实验结果表明NiAlCC-LDHs是处理含氟水的潜在高效吸附剂。
对材料的除氟性能影响最大,是因为阴离子所带电荷越高,与吸附剂表面的静电引力越大,吸附越牢固。实验结果表明NiAlCC-LDHs是处理含氟水的潜在高效吸附剂。

NOTES
*通讯作者。