1. 引言
结构多样性和复杂性的化合物的合成是现代药物和有机合成的热点领域。而多组分反应(multi-component reaction, MCR)正是可以实现快速大量地合成具有结构多样性和复杂性的化合物和建立相应的化合物库[1] [2] ,所以引起了药物化学家的极大关注。目前多组分反应已经在药物发现、农药化学和天然产物全合成等方面得到广泛应用[3] -[5] ,成为有机合成化学家们手中的一个有力工具。
以异腈为原料之一的多组分反应是研究最多的多组分反应[6] -[9] ,由于它们与其他的多组分反应相比更具功能性和多样性,广泛引起有机化学工作者的研究兴趣。对于多组分反应的发展异腈最大的潜力在于成键的多样性,它们的官能团忍耐性以及较高的化学、区域和立体选择性。异腈参与的多组分反应的主要活性位点是异腈官能团,这个官能团能和亲核试剂、亲电试剂发生反应生成α-加成物,异腈其他的主要反应途径有自由基反应,α-酸性以及对于金属有机试剂固有的高亲核能力及反应活性。迄今为止,在合成化学和医药化学起着重要作用的以异腈为原料之一的多组分反应主要是Ugi反应[10] 和Passerini反应[11] 。
2. 异腈参与的Ugi多组分反应
2.1. 简单的Ugi反应
2.1.1. 无催化剂的Ugi反应
虽然Ugi反应的发现已有几十年,但是由于其构建骨架结构的强大功能以及产生大量药物试剂的作用,使得化学工作者乐此不疲地从事这项研究。Fulop等[12] 成功利用四中心–三组分的Ugi反应生成脂环的β-环酰胺(Equation (1))。
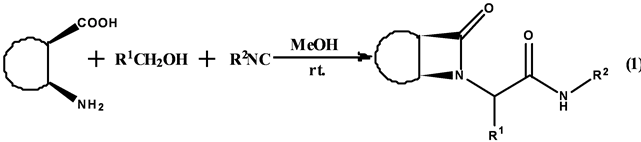
该课题组利用含顺式脂环的β-氨基酸、羰基化合物和异腈反应生成β-环酰胺。反应以甲醇为溶剂,收率最高可达81%,反应具有高非对映选择性,dr值最高可达10:1。在优化的条件下,含有供电子基团或吸电子基团的芳香醛或者甲醛都有中等到较好的收率和较高的非对映选择性。
Oskressensky等[13] 利用酮、叠氮化钠、氯化铵以及相应的异腈生成苯二氮卓类衍生物(Equation (2))。
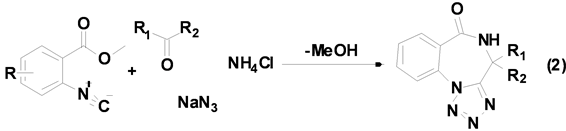
苯二氮卓类化合物具有很好的药物活性和生物活性,是重要的药物中间体,利用Ugi-4CR合成此类化合物时,反应底物羰基化合物仅限于酮,产率也只在32%~81%之间,而醛在此条件下根本不发生任何反应。以前的文献资料主要围绕双功能试剂来完成此类反应,但这是第一次利用异腈参与的多组分反应来合成苯二氮卓类衍生物。目前该小组正在研究醛参与的此类反应,并取得了一些可喜的结果。
Shaabani等[14] 人利用水杨醛、Meldrums酸、异腈以及芳香酚或者脂肪醇较高产率合成了系列3,4-二氢香豆素衍生物(Equation (3)),这个反应可用简单的“一锅法”合成,反应中无需加入催化剂,在室温下即可实现。反应以甲醇或者乙醇作溶剂时比在二氯甲烷中进行效果明显的多,最高产率可达96%,可立体专一性的得到某种顺式异构体,同时这个反应有很好的官能团适用性,产物有很高的产率且易分离。

同时该小组也对该反应提出了一个可能的机理,首先水杨醛和Meldrums酸通过Knoevenogel缩合反应得到中间体化合物5,随后化合物5通过与异腈进行Micheal加成生成中间体6,随后发生分子内的亲核取代反应生成中间体7,然后7在醇的作用下发生亲核加成反应生成中间体8,同时发生分子内开环反应得到香豆素类衍生物4(Scheme 1)。
随后该小组[15] 又报道了一种利用脂肪族或芳香族的异腈与酸酐和丁炔二酸酯的Ugi四组分反应生成多取代呋喃衍生物,产率可达85%,为呋喃衍生物的合成提供了一种简便的方法,同时研究了其在药物合成以及天然产物全合成方面的应用(Equation (4))。

在优化的实验条件下,该反应对于不同结构的异腈以及丁炔二酸酯均有很好的适用性,同时也很好

Scheme 1. A plausible mechanism to form the 2,3-dihydrocoumarins from salicyaldehydes, Meldrums acid, isocyanides and alcohols
Scheme 1. 水杨醛,Meldrums酸,异腈和醇的四组分反应合成3,4-二氢香豆素衍生物可能的反应机理
的解决了复杂产物合成中使用昂贵的催化剂或对环境不友好的催化剂带来的问题,目前该反应的机理仍在进一步研究中。
2.1.2. 酸催化的Ugi反应
在杂环化合物的合成和一些含有杂原子的药物中间体的合成中,特别是含氮杂环化合物的合成中,异腈参与的Ugi多组分反应具有潜在的优越性,可以利用“一锅煮”的方法快速实现该类化合物的合成,其中酸催化的Ugi多组分反应目前受到药物学家和有机合成化学家的广泛关注。
Shaabani课题组[16] 利用异腈与2,3-二氨基丁烯二腈、酮在催化剂对甲基苯磺酸的作用下,最高可以98%的产率得到六元含氮杂环二氢吡嗪衍生物(Equation (5))。
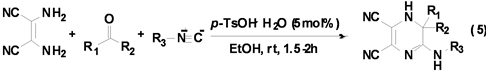
反应是在乙醇溶剂,室温下进行的,在优化的条件下底物的适用范围较广,该反应对于不同的酮和异腈均有很好的产率(80%~98%)。
随后该课题组[17] 又拓展了该反应将底物换成了邻苯二胺类化合物,并以高于95%的产率合成得到苯并二氢吡嗪类衍生物,进一步拓展了该类反应的应用范围,并建立了相应的化合物库(Equation (6))。

同年,该课题组[18] 发现了通过2-氨基苯甲酰胺、醛以及异腈在催化剂对甲苯磺酸的作用下可以高产率的得到酰胺类衍生物。该反应在乙醇作溶剂,室温下即可进行,产率可高达>90%(Equation (7))。

2.2. Ugi反应的拓展
应用双官能团试剂参与的多组分反应可以合成结构多样性、复杂性的具有生物活性或药物活性的化合物。异腈参与的Ugi多组分反应在这类化合物的合成中具有一些其他合成方法所不具有的优越性,如原子利用率高、反应条件温和、产物后处理简单,符合绿色化学的要求,近年来一些异腈参与的新型Ugi多组分反应被发现,并得到快速发展。
2.2.1. Ugi-Smiles反应
异腈参与的Ugi反应多涉及到羧酸类化合物,近年来苯酚参与的有机合成反应受到诸多科研工作者的关注,若将Ugi反应底物中的羧酸变成了苯酚,随后的Mumm重排反应相应的就变成Smiles重排,进而发展了一类Ugi-Smiles反应。
Laurence[19] 等人通过利用Ugi-Smiles反应成功合成了一类含有嘧啶环和多个氮原子的化合物。该反应利用芳醛、异腈、烯胺以及4-羟基嘧啶在甲苯中加热到110℃反应12 h以上即可生成目标产物,随后通过Ru催化生成相应的二环多元杂环化合物(Equation (8))。

利用此反应亦可合成具有特定结构的七元或八元环杂环化合物,同时利用随后的稀土配合物催化剂的催化环化反应可以合成得到更加稳定的异构化产物。
苏州大学的纪顺俊课题组[20] 报道了利用偕二腈取代的烯烃、异腈以及苯并噻唑硫醇反应合成得到具有两个相邻手性中心的亚胺–吡咯烷–硫酮类化合物。该反应具有很好的对映选择性,反应条件温和,在混合溶剂乙腈–水(V:V = 3:1)中,室温下搅拌5 h,即可以高达96%的产率得到产物(Equation (9))。

通过对反应的研究,发现含有拉电子基团的二腈烯类化合物可以在较短的时间内高产量的得到产物。而含有给电子取代基的二腈烯类化合物反应时间提高到24 h也只得到微量产物。作者对反应提出了一个可能的机理(Scheme 2),首先是偕二腈与异腈反应,随后硫醇进攻产生的中间体9,紧接着发生Ugi-Smiles重排得到中间体A,最后通过氨基亲核加成反应得到最终产物。
2.2.2. Ugi-Diels-Alder反应
Diels-Alder反应是有机化学中一个基础而且重要的反应,它在构建环状化合物的过程中相当实用。通过将它与Ugi多组分反应相结合为药物中间体的合成提供了一个方便、快捷的方法,经常是先通过Ugi反应将多个组分会聚起来,再通过分子内的Diels-Alder反应构建具有药物活性的环状化合物结构。
浙江大学的黄宪课题组[21] 发展了一种利用呋喃甲醛、胺、2-苯硒代丙烯酸与异腈的四组分反应构建了含有苯并吡咯酮骨架的杂环化合物(Equation (10))。

该反应在甲醇中通过BF3-OEt2促进,经历一个Ugi四组分反应和分子内的Diels-Alder反应实现杂环化合物骨架的构建,并且反应后处理简单。
2.2.3. 其他新型Ugi多组分反应
Aguiam, N. R.等[22] 基于固相支载的功能化异腈树脂的Ugi四组分反应合成得到α,α-二烷基甘氨酸衍生物(Equation (11)),该反应的不足之处在于生成中间体α,α-二烷基酰胺的反应时间较长(3~15 days),最终

Scheme 2. Possible mechanism for the Ugi-Simles reaction of gem-dicyano olefins, isocyanides, and 2-mercaptobenzothiazoles
Scheme 2. 偕二腈,异腈和硫醇的三组分Ugi-Smiles反应机理
目标产物产率中等至良好(58%~82%)。因此,该反应条件需进一步优化,期望能在较短时间内以更高产率合成目标产物。

Tron课题组[23] 发展了一种新颖的基于异腈、胺和顺式氯肟的三组分反应合成得到系列α-肟基脒衍生物的方法(Equation (12)),该反应无需加入金属或有机催化剂,在室温条件下即可快速进行,反应条件温和,实验后处理简单。同时文中进一步指出该反应在发展新颖异腈与肟的多组分反应中应具有重要的作用。

青岛科技大学文丽荣课题组[24] 发展了一种基于异腈、α-氰基酰腙和丁炔二羧酸酯的三组分反应区域性选择合成多取代吡啶-2-酮衍生物的方法(Equation (13)),该反应具有原料来源广泛,操作简单,条件温和,底物适用范围广和区域选择性高等优点。该反应虽然条件温和,但是反应时间长达20 h以上,为提高反应效率和产率,可以加入催化剂以缩短反应时间,提高目标产物产率。
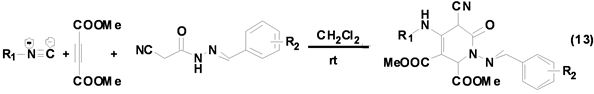
呋喃及其衍生物是重要的杂环化合物骨架,广泛存在于天然产物中,具有较好的生物活性。Mehdi Ghandi[25] 等基于脂肪族异腈、喹啉甲醛和丁炔二酸酯Ugi四组分反应合成系列多取代呋喃衍生物(Equation (14)),产物产率高达95%。该方法操作简单,条件温和,为多取代呋喃衍生物的合成提供了一种简单、高效的合成方法。

Ebrahim Soleimani[26] 等研究了基于水杨酸、丙二腈、伯胺和异腈的四组分反应,结果表明,当反应溶剂及底物加入顺序改变时可以得到两种结构不同的产物。当四种底物同时加入反应瓶,以乙醇作溶剂时,可以中等以上产率一锅合成得到苯甲酰胺衍生物(Equation (15))。

如果将溶剂换成二氯甲烷,且在反应瓶中加入水杨酸、丙二腈和异腈,反应一段时间后再加入伯胺,可以良好以上产率得到一种异吲哚螺吡咯-2-酮衍生物(Equation (16))。该多组分反应显示了对底物的高度适应性,具有实验条件温和,后处理简单等优点,在一些药物分子的设计合成中有重要的应用价值。

3. 异腈参与的新型Passerini多组分反应
近年来异腈参与的多组分反应得到了迅速发展,不断有新型异腈参与多组分反应被发展,除上述介绍的Ugi多组分反应外,另一类研究较多、发展较快的异腈参与的多组分反应是Passerini多组分反应,主要用于合成含杂原子的直链化合物。
3.1. 无催化的新型Passerini多组分反应
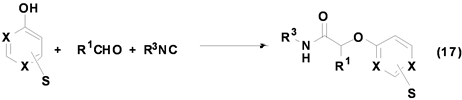
Grimaud[27] 等利用嘧啶酚与醛和异腈反应,成功合成出酰胺类衍生物(Equation (17))。该反应可在甲醇中进行,底物的适用范围较广,无论是脂肪族醛或芳香族醛均能以中等以上产率得到产物,即使是α,β-不饱和醛也等以较好产率得到产物,且该反应目前该课题组仍在进一步研究中。
Soeta等人[28] 首次报道了三苯基硅醇参与的Passerini多组分反应,并以较高产率合成得到硅醚类衍生物(Equation (18)),该反应的底物适用范围较广,各种醛和异腈均可反应,产率良好。
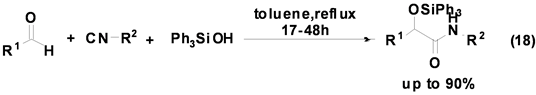
3.2. Lewis酸催化的新型Passerini多组分反应
Taguchi等[29] 报道了一个以Lewis酸In(OTf)3作催化剂,催化α,β-不饱和醛、醇和异腈的三组分反应合成得到α-烷氧基酰胺类衍生物(Equation (19)),反应产率良好,产物后处理简单。
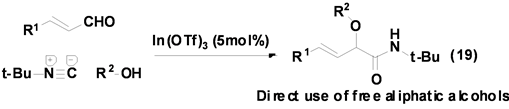
祝介平课题组[30] 报道了CuCl2催化异腈、醇和羧酸衍生物的三组分反应合成得到α-酰胺基酯类衍生物,产率良好(Equation (20))。该反应在甲苯中只需加入催化量的CuCl2、TEMPO、NaNO2,并在氧气中即可顺利的进行。为提高该反应的产率,该课题组仍在进一步研究各种催化剂对该类反应的催化活性和选择性。
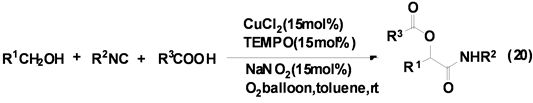
3.3. 其他新型Passerini多组分反应
Basso[31] 等人将微波技术应用到多组分合成中,发展了微波促进的Passerini-Zhu多组分反应,合成得到5-酰胺噁唑啉衍生物(Equation (21))。该反应具有反应时间短、实验操作简单、后处理容易以及产率高等特点,最高产率达到99%。

Meier等[32] 将Passerini多组分反应引进了聚合物合成中,该方法通过Passerini-3CR反应将聚合物单体通过多种形式进行键合,再经多种聚合反应可以合成得到一种性能优异的高分子材料(Equation (22)),进一步拓展了Passerini-3CR反应的应用范围。为聚合物的简便合成提供了一条便捷的方法。

Massoudi, A.等[33] 首次报道了基于SiO2-nanoparticles (NPs)催化的Passerini-Smiles三组分反应合成得到系列α-芳氧基酰胺衍生物(Equation (23))。该反应条件温和,操作简单,首次将纳米Si催化剂应用于异腈参与的Passerini-Smiles三组分反应中,显示了其较高的催化活性和催化选择性。
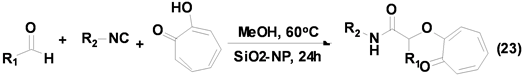
氧杂环丁烷衍生物广泛存在于一些药物分子中,成为药物化学领域研究的热点,引起有机化学家和药物学家的广泛关注。Beasley等[34] 发展了一种Passerini-3CR合成系列3,3-二取代氧杂环丁烷衍生物的方法(Equation (24)),该反应以氧杂环丁酮、异腈和羧酸为基本原料,室温下以优良产率合成得到系列3,3-二取代氧杂化环丁烷的衍生物,但是该反应仅对脂肪族取代异腈适用,因而限制了其应用。
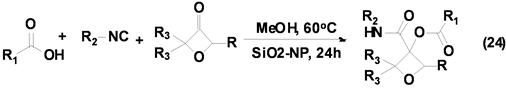
吲哚环广泛存在于天然产物和药物分子中,因其具有较好的生物活性和药物活性而引起有机化学家的广泛关注,近年来基于颠红的有机反应有大量文献报道。Esmaeili等[35] 报道了基于4Å分子筛催化的颠红、羧酸和异腈的Passerini三组分反应合成系列3,3-二取代吲哚-2-酮衍生物(Equation (25))。研究了底物取代基的电子效应的影响,结果表明,该反应对颠红和羧酸的适用性很强,脂肪族羧酸比芳香族羧酸的反应活性高,颠红苯环上连有吸电子基时对反应有利。因此该反应为吲哚衍生物的合成提供了一种简便、高效的方法,在医药领域有重要的应用。

Soeta, T.等[36] 对一种新型的O-Silylative Passerini多组分反应进行了研究,该反应以硅醇为诱导,促进异腈与醛的加成–重排反应生成α-硅氧基酰胺(Scheme 3)。
基于以上机理,作者研究了具有亲电–亲核基团的双亲体(如二苯基硼酸等)促进的醛、异腈和水的三组分加成反应,基于研究结果,作者提出了一种催化循环机理(Scheme 4)。首先,活化的硼原子对醛基原子加成,继而异腈中亲核性的碳原子进攻羰基碳原子形成氮杂炔正离子中间体D,然后有两种途径可以完成催化循环过程。path a途径指出中间体D中硼酸中羟基进攻炔基中碳原子继而发生重排生成中间体F,发生水解反应放出催化剂二苯基硼酸得到最终产物。path b途径中,水先进攻中间体D中炔基碳生成中间体G,继而失去质子得到中间体酰胺H,中间体H再质子化水解生成催化剂二苯基硼酸和目标产物。作者认为该催化循环机理以path a为主,主要是因为分子内重排反应比水进攻中间体D的反应更快。
4. 结论与展望
综上所述,异腈参与的多组分反应在合成分子多样性和结构复杂性的化合物方面具有独特的优势,在合成一些具有生物活性或药物活性的杂环化合物方面更是如此。异腈参与的多组分反应的研究将随着科学的发展不断深入,特别是在手性催化合成方面有较大的发展前景,利用有机小分子、过渡金属、手
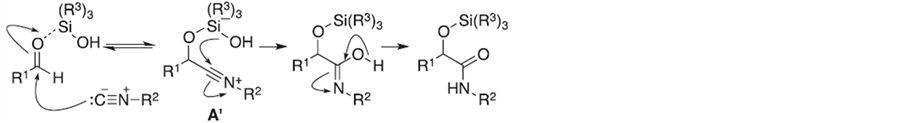
Scheme 3. Plausible reaction mechanism of the O-silylative Passerini reaction
Scheme 3. 硅醇促进的Passerini多组分反应机理

Scheme 4. Proposed catalytic cycle for α-addition of isocyanides catalyzed by borinic acids
Scheme 4. 双亲体促进的醛、异腈和水的三组分反应催化循环机理
性催化剂等催化的Ugi多组分反应、Passerini多组分反应等将是未来有机化学家今后研究的热门领域。
基金项目
国家自然科学基金(No. 21172001, 21372008),教育部新世纪优秀人才计划(No. NCET-10-0004)和安徽省自然科学基金(No. 1308085QB39)资助项目。

NOTES
*通讯作者。