1. 引言
在稀土元素家族中铈是在自然界中丰度最高和最为廉价的元素,二氧化铈是一种用途极广的稀土氧化物,广泛应用于工业材料。纳米CeO2和铈基纳米材料因具有独特的晶体结构和特殊的4f电子结构,使其具有很好的储氧、释氧、光学、磁学等性能,可广泛用于燃料电池、催化剂、抛光材料、氧化物传感器、电子陶瓷、紫外吸收剂材料[1] -[6] 等。不同形貌的纳米CeO2对其所具有的性能的发挥有着显著的影响,不同的制备方法及不同的制备条件对其制备样品的形貌也有着不同的影响,许多学者一直致力于通过研究不同的制备方法及制备条件制得形貌可控的CeO2纳米粉体。目前制备纳米CeO2的主要方法有固相法、液相法、气相法。液相法主要有化学沉淀法[7] 、溶液–凝胶法[8] 、水热法[9] 、微乳液法[10] 等。水热法作为液相法中的一种,是在密闭的压力容器中,以水或其他液体作为介质,在高温高压等条件下制备优质氧化物或化合物粉体的一种湿化学合成方法。由于水热反应是在密闭的高温高压溶液中进行的,因此可得到其它方法难以获取的低温同质异构体,从而获得新的物相。
从已有的研究报道中我们知道,水热法可直接制备出纳米CeO2粉体,制得的产物具有纯度高、粉体细、分散性好、颗粒大小均匀、晶型好、形状可控等特点。由于水热法设备简单,条件温和,而且易于得到特殊形貌的纳米材料,近年来许多学者常用此法来制备形貌可控的纳米CeO2,如:CeO2纳米棒[11] ,CeO2纳米管[12] ,CeO2纳米立方块[13] ,CeO2纳米中空球[14] ,三叉枝状纳米CeO2[15] 等。利用水热法制备形貌可控的CeO2纳米粉体已成为近年来研究的一大热点。
在利用水热法制备纳米CeO2过程中,控制不同的反应条件可制得不同形貌的纳米CeO2样品。不同形貌的纳米CeO2对其性能的发挥有着重要的影响,比如相同尺寸的纳米颗粒,比表面积越大催化性能越强,其中管状、中空球状、三叉枝状等形貌的纳米颗粒的比表面积均比较大,具有很好的催化性能。由此可见,探索合成具有特殊形貌的CeO2有待进一步研究。
水热法在制备形貌可控的CeO2方面显示出较大的潜力,水热或溶剂热体系下合成形貌结构各异、性能优良的CeO2在理论和应用上都具有极为广阔的发展前景。研究各因素对CeO2形貌的影响机制,有助于工业化生产,合成高质量、高性能的CeO2产品,从而带来巨大的经济效益,基于理论和实验通过水热法制备出形貌可控的CeO2来提升其性能对未来的工业和科技的发展都有重要意义,是亟待解决的问题,也是CeO2未来重点研究的方向之一。
通过水热法合成的纳米CeO2粉体的形貌受溶剂、表面活性剂、铈源、反应时间、反应温度等因素的影响。赵晓兵等[16] 以七水氯化铈为铈源,P123为表面活性剂,利用水热法合成CeO2纳米管,研究反应时间、反应温度对CeO2纳米颗粒形貌的影响。研究发现,随着时间的延长,CeO2形貌也在发生着演变,通过控制反应时间能够合成不同形貌的纳米二氧化铈产品。在现代工业生产过程中,温度是最易控制的物理量之一,研究温度在利用水热法合成不同形貌纳米二氧化铈过程中的作用机理,有利于控制合成不同形貌的纳米二氧化铈产品,获得不同性能用途的高品质产品,而且采用控制温度法合成不同形貌的二氧化铈产品,简化生产工艺,有利于降低生产成本。已报道的文献中,鲜有研究在酸性条件下利用水热法通过控制反应时间来调控纳米二氧化铈形貌。本实验采用水热法在酸性条件下合成纳米CeO2,并研究不同反应时间下纳米CeO2的合成与表征。
2. 实验部分
2.1. 合成
实验所用试剂有六水合硝酸铈(分析纯),氨水(分析纯),硝酸(分析纯),蒸馏水(自制)。实验步骤为:将1.73688 g Ce(NO)3·6H2O溶解于60 ml蒸馏水中,再加入6 ml氨水,并用磁力搅拌器搅拌3 h后缓慢滴加稀H2NO3调节PH至酸性后置于高压反应釜中,放入烘箱内在200℃条件下分别反应8小时、12小时、16小时,反应结束后自然冷却,取出,先水洗后醇洗各三遍,再用烘箱在80℃条件下烘干备用。
2.2. 二氧化铈的表征
采用X射线衍射仪(Cu Kα λ = 0.15408 nm,扫描范围2θ = 10˚~90˚)分析CeO2产物的物相;在激光显微共聚焦拉曼光谱仪上测定CeO2产物的拉曼光谱;样品的形貌,粒度大小等微观结构在扫描电子显微分析仪(SEM)上观察;通过紫外可见分光度计测定Eg值。
3. 结果与讨论
3.1. 纳米二氧化铈样品的X射线衍射
图1是利用水热法在不同的反应时间下制得产物的XRD图谱,A、B、C分别代表反应时间为8小时、12小时、16小时。从图中可观察到,所制得产物的XRD图与标准粉末衍射卡(JCPDS NO.81-0792)相吻合,衍射指标化表明产物为萤石结构立方相的二氧化铈晶体,其晶格常数借助软件分析得8小时条件下制得的样品晶格常数为5.4177 nm,12小时条件下制得的样品晶格常数为5.4224 nm,16小时条件下制得的样品晶格常数为5.4233 nm,在XRD衍射图中没有观察到其它杂质峰,说明产物晶化程度较高。由图还可知,随着反应时间的增加,峰的强度越来越强,其衍射峰的半峰宽度也逐渐变窄,由Scherrer公式 ,且在不考虑由仪器等引起的宽化时,我们知道晶粒尺寸与半峰宽成反比关系,即说明产物的粒径大小随着反应时间的升高而略有增大。另一方面,衍射曲线的平滑程度也与晶格发育状况有关,其晶格发育较为完整的粉末,衍射曲线的特征峰均十分平整、光滑,当发育不完整时,衍射曲线就会出现许多毛刺,说明颗粒的晶型发育不够完好,结晶不够完全。由衍射图可见,随着反应时间的增加,衍射曲线略成平滑趋势,有利于晶型的发育,即说明,随着反应时间的增加,二氧化铈的结晶会更好。
,且在不考虑由仪器等引起的宽化时,我们知道晶粒尺寸与半峰宽成反比关系,即说明产物的粒径大小随着反应时间的升高而略有增大。另一方面,衍射曲线的平滑程度也与晶格发育状况有关,其晶格发育较为完整的粉末,衍射曲线的特征峰均十分平整、光滑,当发育不完整时,衍射曲线就会出现许多毛刺,说明颗粒的晶型发育不够完好,结晶不够完全。由衍射图可见,随着反应时间的增加,衍射曲线略成平滑趋势,有利于晶型的发育,即说明,随着反应时间的增加,二氧化铈的结晶会更好。
3.2. 纳米二氧化铈样品的SEM表征
图2为不同的反应时间下纳米二氧化铈的SEM图。由于纳米粒子的粒径细小,表面活性能极高,且极为不稳定,很容易与其它粒子相结合,而导致团聚现象的发生,然而由于该实验中未添加任何表面活性剂,故不能很好的消除团聚现象,但我们还是能从图中观察到,不同反应时间条件下所制得的产物的分散性和产物形貌略有不同。由图观察可知,该反应条件下制得的纳米二氧化铈产物呈球形颗粒,且随着反应时间的增加,所制得的产物分散性逐步变好,但反应时间过长反而降低产物的分散性,增加团聚。
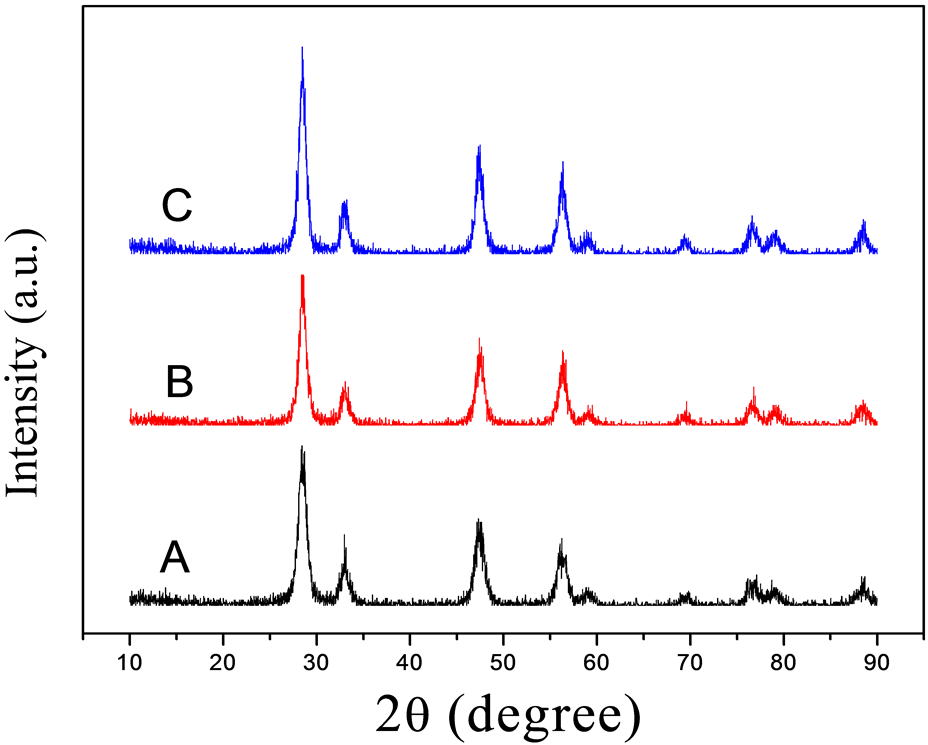
Figure 1. XRD patterns of the samples under the reaction time of (A) 8 h, (B) 12 h, and (C) 16 h
图1. (A) 8 h,(B) 12 h和(C) 16 h的反应时间下样品的XRD图谱
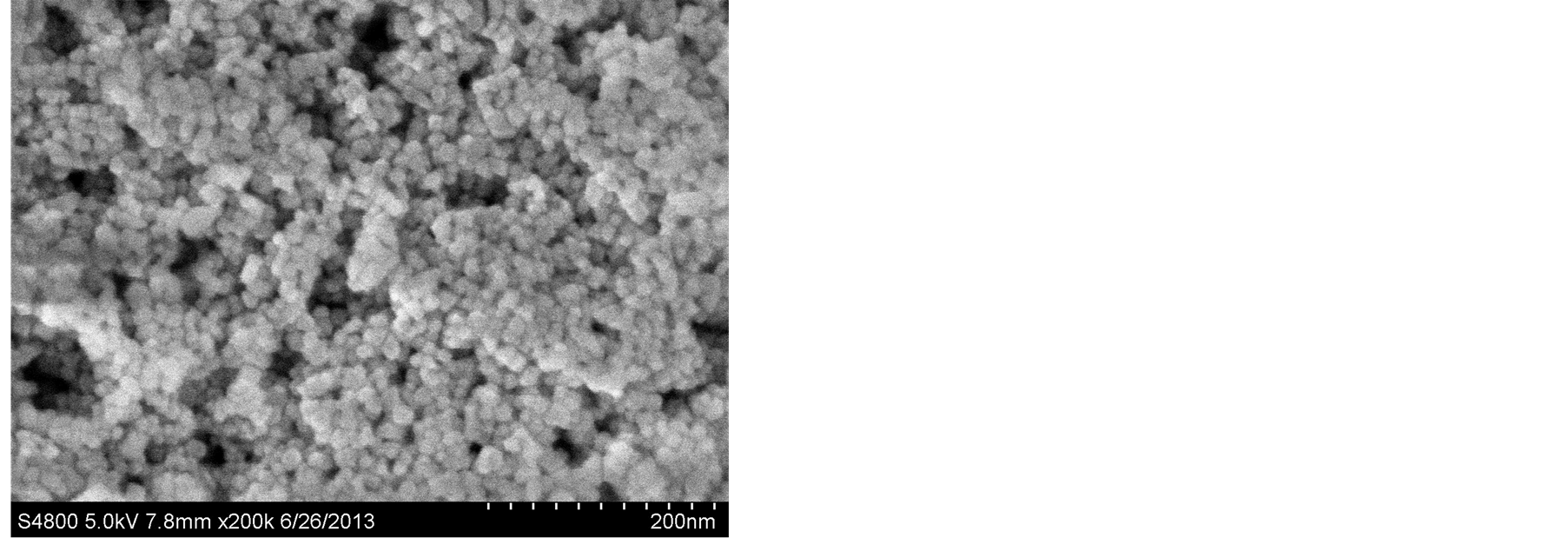 (a)
(a) (b)
(b)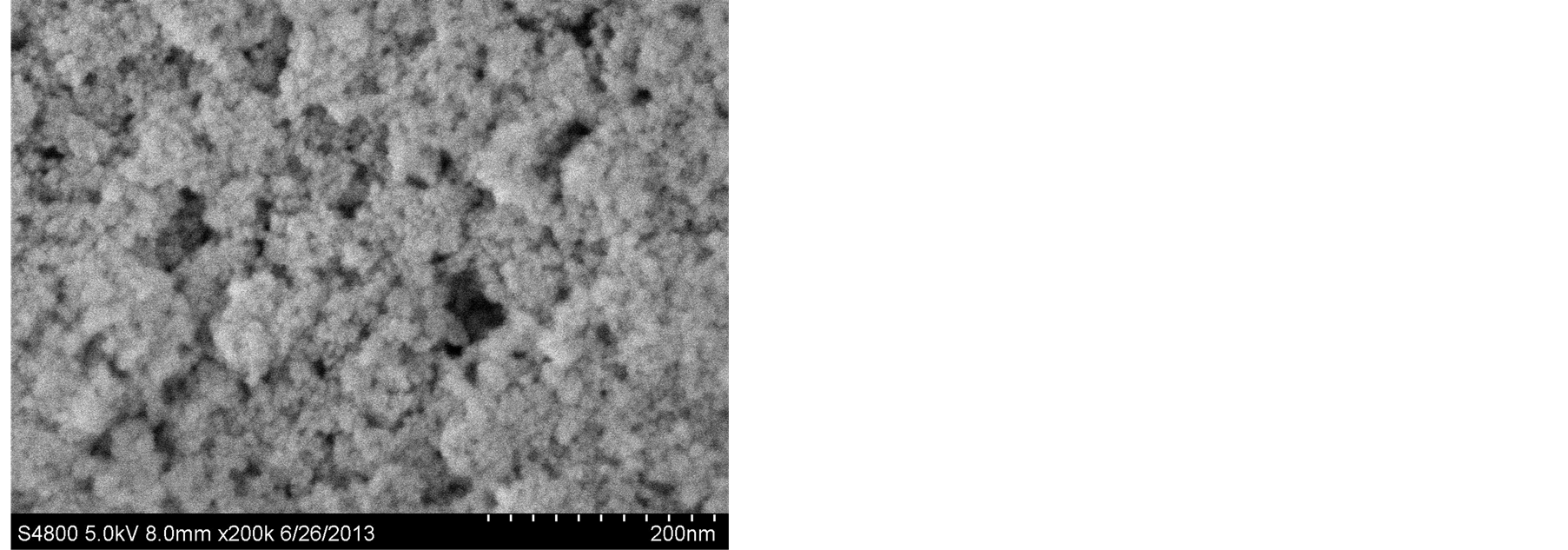 (c)
(c)
Figure 2. SEM images of the samples under the reaction time of (a) 8 h, (b) 12 h, and (c) 16 h
图2. (a) 8 h,(b) 12 h和(c) 16 h的反应时间下样品的SEM图
从实验结果我们分析可知,在适当延长反应时间的条件下,我们能够制得分散性较好,团聚现象较低的球形纳米二氧化铈晶体,但反应时间过长不利于获得分散性良好的纳米二氧化铈产品,实验表明,最佳的反应时间为12个小时且反应温度控制在240℃。
在合适的反应温度条件下,利用其提供的激活能,小晶体或晶核通过表面成核相互垒叠或侧向连接组合成颗粒状,由此形成形状不规则的颗粒。在一定的反应时间内,随着反应时间的增加,反应进行的比较充分,产物在反应过程中吸收足够的激活能,用于升高其表面能,降低团聚,形成颗粒较为分散的CeO2纳米颗粒。但反应时间超过一定的限度,纳米CeO2颗粒将沿着某一方向继续生长,由于本实验中未添加分散剂的原因大量的小晶体或晶核继续通过表面成核相互垒叠或侧向连接组合大成颗粒,造成反应物的团聚现象,分散性变差,产物的颗粒尺寸变大。
3.3. 纳米二氧化铈样品的激光拉曼光谱图
图3中A、B、C分别为反应时间为8小时、12小时、16小时的条件下的拉曼谱,由文献资料知,对空间群为Fm3m的面心立方萤石结构型,唯一的拉曼活性模式是F2g,萤石结构二氧化铈的该拉曼信号在465 cm−1。由拉曼谱图知,本文研究所制得的二氧化铈样品均在462 cm−1有很强的拉曼峰,应归属于Ce-O-Ce对称伸缩振动的F2g模式。但该峰在不同的反应时间下会因晶体结构的细微变化而偏移465 cm−1值较文献报道[17] 的略向低波数移动,可能是由于粒径较小、晶格发育不完全的缘故产生此现象。
由图可知,随着反应时间的不同,拉曼谱的主峰强度略有不同。反应时间为12小时条件下拉曼峰最为强烈,且在1080 cm−1左右出现一个略微明显的次峰。随着反应时间的改变拉曼谱出现峰值的波数位置基本上不改变,只是峰值略有不同。
3.4. 纳米二氧化铈样品的紫外吸收谱分析
图4中A、B、C分别表示反应时间为8小时、12小时、16小时下的带隙图。纳米材料的光学吸收性能与其纳米结构的形貌、尺寸、材质本身等诸多因素有关。对于CeO2半导体的光吸收谱,其带边区域的光吸收值满足下列公式:(αhv)n = A(hv-Eg),其中α为吸收系数;hv为入射光子能量;Eg为光学带隙;对于允许的直接带隙n = 1/2,对于禁介的直接带隙跃迁n = 3/2,对于间接带隙跃迁n = 2。由图4可知:不同反应时间下的Eg值不同,反应时间为8小时、12小时、16小时所对应的Eg值分别为2.75 eV、2.65 eV、3.01 eV,和文献报道[18] 块状二氧化铈相比,8小时条件下和12小时条件下制得的样品其光学带隙稍有蓝移,而16小时条件下制得的样品稍有红移,可能是晶体表面存在缺陷以及晶体的发育不够完好有
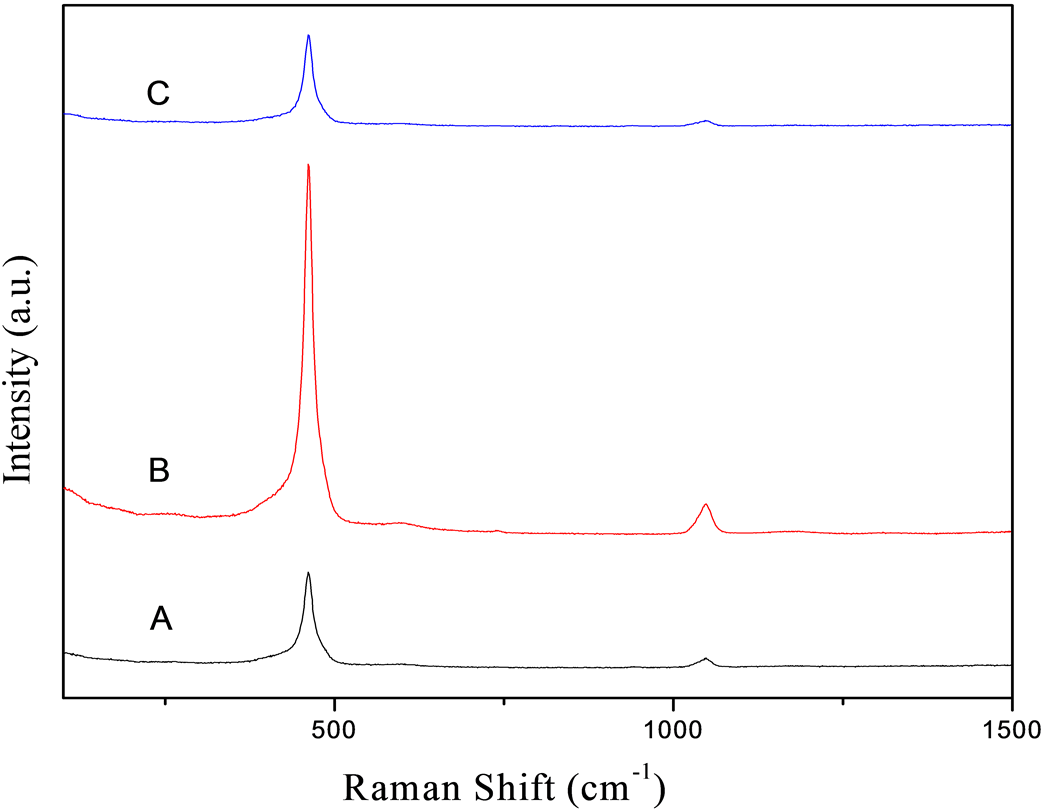
Figure 3. Raman spectra of the samples under the reaction time of (A) 8 h, (B) 12 h, and (C) 16 h
图3. (A) 8 h,(B) 12 h和(C) 16 h的反应时间下样品的拉曼谱
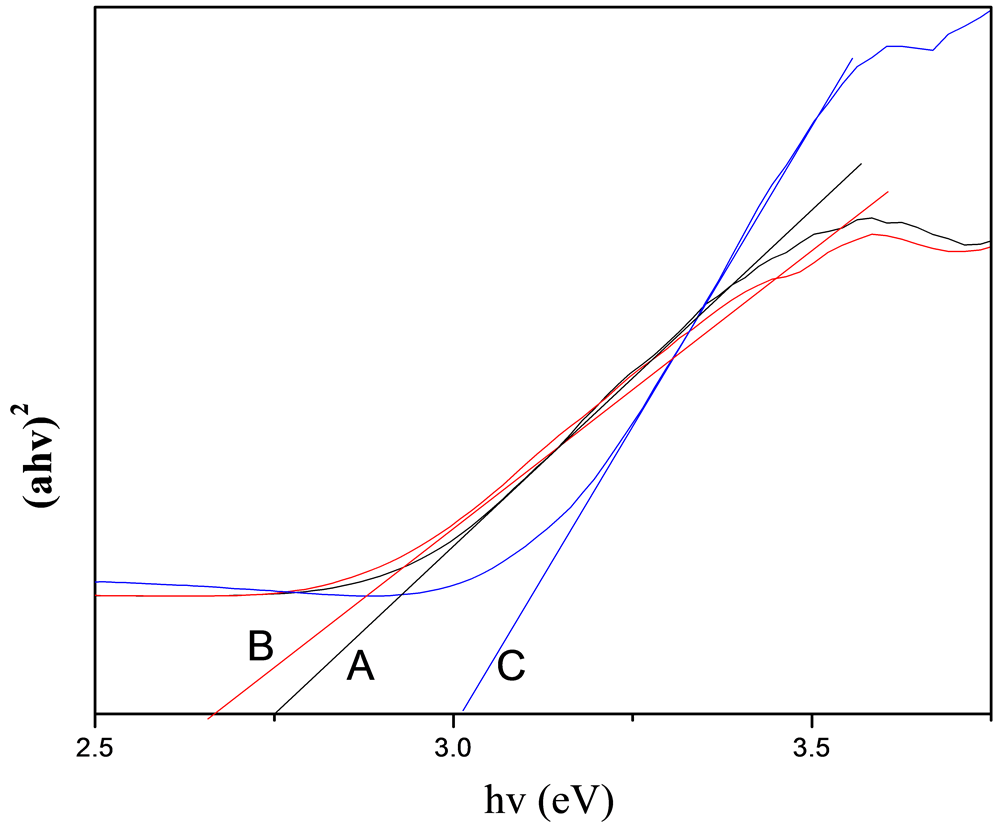
Figure 4. Plots of (αhv)2 as function of energy of the samples under the reaction time of (A) 8 h, (B) 12 h, and (C) 16 h
图4. (A) 8 h,(B) 12 h和(C) 16 h的反应时间下样品的带隙图
关。由分析可知,不同反应时间条件下制得的纳米二氧化铈样品的带隙值差别明显,通过控制反应时间条件就能够制备出所需大小的带隙纳米二氧化铈产品,实验操作简单,易于实验操作,也适合工业化生产。二氧化铈产物在紫外区有很强的吸收峰,该反应条件下所制得的产物也可作为紫外吸收材料使用。
4. 结论
本文采用生产成本低的水热合成法制备纳米二氧化铈粉体,以硝酸铈为铈源,硝酸为酸性控制剂,在酸性条件下进行反应,着重研究反应时间对产品形貌和性能的影响。初步得出制备形貌可控纳米二氧化铈的实验工艺条件,实验表明在反应时间为12小时的条件下产物的分散性、形貌最佳,为最佳的反应时间。在反应温度、PH、反应液浓度等最佳条件下,通过改变反应时间制备形貌可控的纳米二氧化铈粉体,研究发现在一定的反应时间范围内,随着反应时间的增加,样品的颗粒粒径随着反应时间的增加略有增大,超过一定的反应时间会增加产物的团聚现象,分散性变差。酸性条件有利于抑制铈离子的水解反应发生,降低氢氧化铈的产量,通过本研究能够初步探索出在酸性条件下的最佳的反应时间为12小时,该反应条件下产物的质量最好。综上所述,本实验所作出的结论为制备纳米二氧化铈的工业化生产提供了初步的理论基础。
基金项目
安徽大学2014年创新训练计划项目(201410357127)。

NOTES
*通讯作者。