1. 引言
众所周知,3,4-二氢嘧啶-2(1H)-酮/硫酮化合物具有抗过敏、降压、杀菌、消炎、抗病毒 [1] - [6] 及抑制有丝分裂驱动蛋白 [7] [8] 等重要的药理和生物活性。100多年前,意大利化学家Biginelli首次提出了浓盐酸催化苯甲醛、尿素和乙酰乙酸乙酯三组分反应合成3,4-二氢嘧啶-2(1H)-酮/硫酮的方法 [9] 。然而该方法存在条件苛刻、反应时间长(18 h)且产率低(20%~50%)的缺点。因此,各种催化体系被用于该反应以改进经典方法的不足,如多组分聚合物1,4-DHP和3,4-DHPM [10] 、纳米共催化剂TiO2-SiO2 [11] 、Cu-EDTA负载的APTMS-Fe3O4@SiO2核-壳体系 [12] 、硅钛铝氧化物MxOy [13] 、微波促进 [14] 等。
离子液体因具有蒸气压低、热稳定性好、毒性低、易于回收等诸多优点,在Biginelli反应中也得到了应用 [15] [16] 。基于本课题组在离子液体合成和催化应用领域的研究基础 [17] [18] ,本文提出了一种Brønsted酸性苯并三唑离子液体催化合成3,4-二氢嘧啶-2(1H)-酮/硫酮的方法。考察了催化剂种类和用量、反应溶剂、反应时间等因素对反应产率的影响,同时对反应底物的普适性进行了研究。此外,还探讨了离子液体的催化循环使用效果。
2. 实验部分
2.1. 试剂与仪器
薄层层析硅胶用GF254硅胶,柱层析硅胶:300-400目(青岛海洋化工厂)。美国Varian inova-400型核磁共振仪(400 MHz, TMS);德国Bruker Equinox 55红外光谱仪(KBr压片);美国HP 1100液相色谱质谱仪;瑞士Büchi B-560型熔点仪。所用试剂均为市售分析纯,用前未经处理。
2.2. 离子液体的合成
离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐的合成如式1所示。将0.20 mol的1-丁基苯并三唑和0.24 mol的1-氯乙酸在90℃搅拌反应36 h,冷却至室温,用乙醚和丙酮(V:V = 2:1, 3 × 20 mL)混合溶剂浸泡洗涤所得的棕色固体,抽滤,所得固体在90℃下真空干燥10 h,即得氯化1-丁基-3-羧甲基苯并三唑 [19] ,白色固体,熔点:148℃~149℃。
在室温下,将0.012 mol三氟乙酸缓慢滴加到0.01 mol氯化1-丁基-3-羧甲基苯并三唑中,滴毕升温至80℃回流反应48 h,得到褐色液体,减压旋除过量的三氟乙酸,残余物在90℃下真空干燥10 h,即得离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐[C2O2BBTA][TFA]。
离子液体[C2O2BBTA][TFA]表征数据:褐色液体,[C2O2BBTA][TFA]:1H NMR (400 MHz, DMSO) δ: 8.79-8.24 (m, 3H), 8.06-7.96 (m, 2H), 5.93 (s, 2H), 5.08 (t, J = 7.1 Hz, 2H), 2.06-1.99 (m, 2H), 1.39-1.31 (m,
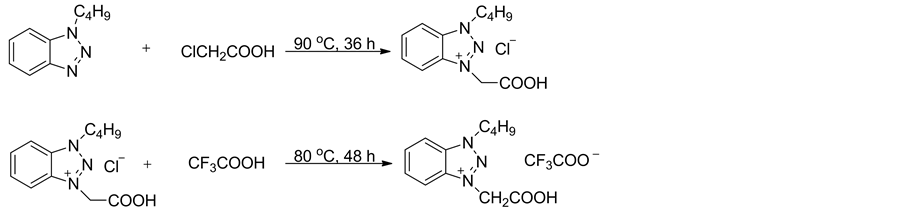
Scheme 1. The synthesis of ionic liquid [C2O2BBTA][TFA]
图式1. 离子液体[C2O2BBTA][TFA]的合成
2H), 0.93 (t, J = 7.4 Hz, 3H), 13C NMR (100 MHz, DMSO) δ: 166.26 134.99, 134.22, 131.13, 130.80, 120.96, 117.76, 114.22, 113.85, 52.66, 51.09, 30.27, 18.81, 13.09. IR (KBr, ν/cm-1): 3106, 2967, 2940, 2879, 2511, 1738, 1505, 1471, 1364, 1190, 1141, 1029, 754, 718, 643, 599. ESI-MS: m/z (%) = 234.1 (100%) [M + H] +.
2.3. 未知化合物4a-4s的合成及结构分析
化合物4a-4r的合成反应如图式2所示。在10 mL圆底烧瓶中加入2 mmol芳香醛、2 mmol β-二羰基化合物和3 mmol脲或硫脲,20 mol%催化剂[C2O2BBTA][TFA],混合均匀后在90℃无溶剂条件下磁力搅拌反应40 min。反应结束后,向混合物中加入大量的碎冰,室温充分搅拌至碎冰融化,过滤即得产物粗品,经过柱层析分离得化合物4a-4r纯品。化合物结构经1H NMR,13C NMR,IR和MS确证结构。
目标化合物的表征如下:
4g:白色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.30 (s, 1H), 7.74 (s, 1H), 7.36 (ddd, J = 15.0, 8.8, 4.4 Hz, 2H), 7.21 (d, J = 2.5 Hz, 1H), 5.60 (d, J = 2.8 Hz, 1H), 3.90 (q, J = 7.1 Hz, 2H), 2.30 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H);
13C
NMR (100 MHz, DMSO-d6) δ: 164.76, 162.01, 159,55, 151.10, 149.30, 138.29, 138.26, 132.28, 130.27, 130.17, 116.34, 116.09, 114.97, 97.63, 58.99, 50.91, 40.02, 17.56, 13.81; IR (KBr, ν/cm-1): 3346, 3225, 3112, 2976, 1697, 1644, 1223, 1093, 903, 805; ESI-MS: m/z (%) = 335.0 (100%) [M +Na] +.
4h:白色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.32 (s, 1H), 7.78 (s, 1H), 7.48 (dd, J = 8.8, 5.2 Hz, 1H), 7.23 – 6.92 (m, 2H), 5.59 (s, 1H), 3.91 (q, J = 7.1 Hz, 2H), 2.30 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H);
13C
NMR (100 MHz, DMSO-d6), δ: 164.71, 162.18, 159.75, 151.00, 149.73, 143.89, 143.83, 131.17, 131.09, 126.86, 116.28, 115.27, 115.03, 97.11, 59.04, 51.76, 17.60, 13.78; IR (KBr, ν/cm-1): 3221, 3098, 2982, 1703, 1650, 1604, 1282, 1237, 1103, 881, 803; ESI-MS: m/z (%) = 335.0 (100%) [M + Na] +.
4i:白色固体;1H NMR (400 MHz, DMSO) δ: 9.30 (s, 1H), 7.79 (s, 1H), 7.49 (dd, J = 6.7, 2.2 Hz, 1H), 7.35 (t, J = 8.7 Hz, 1H), 7.27 (dd, J = 4.9, 2.2 Hz, 1H), 5.15 (d, J = 3.3 Hz, 1H), 4.18 – 3.86 (m, 2H), 2.26 (s, 3H), 1.10 (t, J = 7.1 Hz, 3H);
13C
NMR (100 MHz, DMSO-d6), δ: 165.00, 158.49, 156.06, 151.66, 148.95, 142.90, 142.87, 131.19, 127.48, 127.40, 116.86, 116.64, 107.63, 107.42, 98.42, 59.21, 52.97, 17.72, 13.93, IR (KBr, ν/cm-1): 3342, 3203, 3100, 2984, 1702, 1658, 1232, 1099, 895, 804; ESI-MS: m/z (%) = 379.0 (100%) [M + Na] +.
4m:白色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.15 (s, 1H), 7.70 (s, 1H), 7.24 (t, H), 6.76-6.83 (m, 3H), 5.10 (s, 1H), 4.82 (m, 1H), 3.72 (s, 3H), 2.23 (s, 3H), 1.16 (d, J = 8.0 Hz, 3H), 1.01 (d, J = 8.0, 3H);
13C
NMR (100 MHz, DMSO-d6), δ: 164.73, 159.07, 152.07, 148.05, 146.35, 129.37, 118.20, 112.33, 112.00, 99.34, 66.25, 54.86, 53.73, 21.69, 21.40, 17.60, IR (KBr, ν/cm-1): 3234, 3106, 2981, 2948, 1721, 1652, 1599, 1463, 1431, 1374, 1282, 1232, 1092, 1073, 788; ESI-MS: m/z (%) = 327.1 (100%) [M + Na] +.

Scheme 2. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones (4a-4r)
图式2. 3,4-二氢嘧啶酮/硫酮(4a-4r)的合成
4n:橙色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.32 (s, 1H), 9.07 (s, 1H), 7.59 (s, 1H), 7.02 (d, J = 4.0 Hz, 2H), 6.68 (d, J = 4.0 Hz, 2H), 5.02 (s, 1H), 4.82-4.78 (m, 1H), 2.22 (s, 3H), 1.15 (d, J = 6.4 Hz, 3H), 1.00 (d, J = 6.4 Hz, 3H);
13C
NMR (100 MHz, DMSO-d6), δ: 164.79, 156.39, 152.03, 147.35, 135.47, 127.35, 114.80, 99.92, 66.10, 53.44, 21.69, 21.39, 17.56; IR (KBr, ν/cm-1): 3289, 3227, 3109, 2979, 2808, 1706, 1686, 1651, 1511, 1448, 1371, 1282, 1226, 1173, 1086, 783, 680; ESI-MS: m/z (%) = 313.1 (100%) [M + Na] +.
4o:绿色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.05 (s, 1H), 7.56 (s, 1H), 7.03 (d, J = 4.0 Hz, 2H), 6.65 (d, J = 4.0 Hz, 2H), 5.01 (s, 1H), 4.82-4.80 (m, 1H), 2.84 (s, 6H), 2.22(s, 3H), 1.17 (d, J = 6.0 Hz, 3H), 1.03 (d, J = 6.0 Hz, 3H);
13C
NMR (100 MHz, DMSO-d6), δ: 164.87, 152.17, 149.62, 147.15, 132.80, 126.80, 112.04, 100.12, 66.08, 53.25, 21.72, 21.47, 17.56; IR (KBr, ν/cm-1): 3243, 3116, 2980, 2937, 1719, 1648, 1526, 1457, 1363, 1290, 1231, 1090, 789; ESI-MS: m/z (%) = 340.1 (100%) [M + Na] +.
4p:淡黄色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.10 (s, 1H), 7.67 (s, 1H), 7.22-7.26 (m, 2H), 7.13-7.14 (m, 2H), 5.08 (s, 1H), 2.21 (s, 3H), 1.28 (s, 9H);
13C
NMR (100 MHz, DMSO-d6), δ: 164.61, 162.38, 159.97, 151.81, 147.44, 141.16, 141.13, 128.20, 128.13, 115.03, 114.82, 100.25, 79.10, 53.60, 27.72, 17.56; IR (KBr, ν/cm-1): 3230, 3107, 2975, 2930, 1697, 1644, 1507, 1452, 1366, 1292, 1230, 1164, 1090, 1035, 837, 798, 759, 658; ESI-MS: m/z (%) = 329.1 (100%) [M + Na] +.
4q:淡黄色固体;1H NMR (400 MHz, DMSO-d6) δ: 9.05 (d, J = 1.6 Hz, 1H), 7.64 – 7.63 (m, 1H), 7.23-7.21 (m, 1H), 7.06 – 7.02 (m, 3H), 5.07 (d, J = 3.2 Hz, 1H), 2.28 (s, 3H), 2.21 (s, 3H), 1.29 (s, 9H);
13C
NMR (100 MHz, DMSO-d6), δ: 164.73, 152.06, 147.05, 144.89, 137.07, 128.13, 127.68, 126.80, 123.23, 100.50, 78.99, 54.20, 27.73, 21.01, 17.56; IR (KBr, ν/cm-1): 3226, 3099, 2977, 2935, 1699, 1647, 1489, 1438, 1366, 1294, 1232, 1165, 1087, 859, 813, 774, 745, 697,670,599; ESI-MS: m/z (%) = 325.1 (100%) [M + Na] +.
4r:淡黄色固体;1H NMR (400 MHz, DMSO-d6), δ: 9.08 (s, 1H,); 7.68 – 7.67 (m, 1H), 7.25 (t, J = 8.0 Hz, 1H), 6.83 – 6.78 (m, 3H), 5.08 (d, J = 3.2 Hz, 1H), 3.73 (s, 3H), 2.22 (s, 3H), 1.31 (s, 9H);
13C
NMR (100 MHz, DMSO-d6), δ: 164.74, 159.07, 152.14, 147.29, 146.36, 129.35, 118.14, 112.26, 111.98, 100.35, 79.05, 54.85, 53.95, 27.74, 17.57; IR (KBr, ν/cm-1): 3392, 3247, 3111, 2972, 2937, 1707, 1672, 1519, 1463, 1366, 1282, 1240, 1165, 1094, 1038, 849, 801; ESI-MS: m/z (%) = 341.1 (100%) [M + Na] +.
3. 结果与讨论
3.1. 最优反应条件的筛选
以苯甲醛、乙酰乙酸乙酯和脲三组分反应为模型,考察了催化剂种类和用量、溶剂种类、反应时间等因素对反应的影响。首先考察了2种不同阴离子的1-丁基-3-羧甲基苯并三唑离子液体及相应Brønsted酸三氟乙酸对反应的影响(表1, entries 1-3)。从表中可以看出,离子液体[C2O2BBTA][TFA]的催化活性优于离子液体[C2O2BBTA]Cl和三氟乙酸。其次,考察了催化剂的用量对反应体系的影响(表1, entries 4, 5),发现催化剂用量为20 mol%时,产物产率最高为96%。随后考察了H2O、CH3OH、C2H5OH、i-PrOH、

Table 1. Optimization of reaction conditionsa
表1. 反应条件的优化a
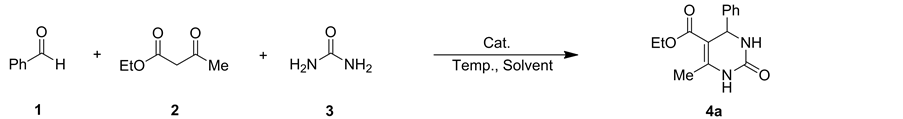
a反应条件:苯甲醛(2 mmol),乙酰乙酸乙酯(2 mmol),脲(3 mmol) %,90℃;b分离产率。
CH2Cl2、CH3CN、DMF、甲苯等八种溶剂及无溶剂条件下反应的效果,发现无溶剂条件下反应效果最佳(表1, entries 6-13)。最后我们对反应时间进行了筛选(表1, entries 14-17),结果表明最佳反应时间是40 min。因此,最优的反应条件为:无溶剂条件下,离子液体[C2O2BBTA][TFA](20 mol%)为催化剂,90℃反应40 min。
3.2. 底物普适性研究
在最优条件下,我们对该反应的底物普适性进行了研究,结果见表2。从中可以看出,苯甲醛的苯环上不管是带有供电子基团还是吸电子基团,都能顺利的参与反应,以82%~98%的收率得到相应的3,4-二氢嘧啶-2(1H)-酮产物(表2, entries 4a-4j)。硫脲代替脲也被用于Biginelli三组分反应,成功地合成了相应的产物(表2, entries 4k, 4l)。使用乙酰乙酸异丙酯、乙酰乙酸叔丁酯代替1,3-二羰基化合物参与反应也能得到令人满意的结果,相应产物的产率为89%~99% (表2, entries 4m-4r)。因此,离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐催化合成二氢嘧啶-2(1H)-酮/硫酮化合物具有很好的底物普适性。
3.3. 离子液体循环使用性
离子液体的特性之一是循环使用,本文以苯甲醛、乙酰乙酸乙酯和脲三组分反应为模型,在最优条件下考察了离子液体催化剂1-丁基-3-羧甲基苯并三唑三氟乙酸盐的循环使用效果。具体方法为:将反应结束萃取分离的水相减压旋除水,残余物经真空干燥至恒重,即得回收的离子液体[C2O2BBTA][TFA],可直接用于下一次催化循环。从图1可知,离子液体催化剂1-丁基-3-羧甲基苯并三唑三氟乙酸盐循环使用4次后仍能保持较好的催化活性,表明该离子液体具有较好的循环使用效果。

Table 2. Investigation of substrate scopea
表2. 底物普适性研究a
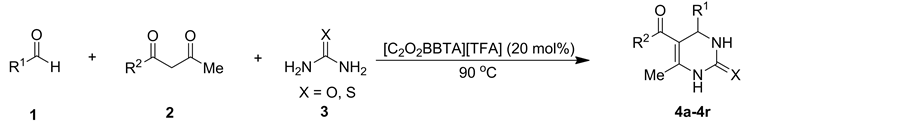
a反应条件:芳香醛(2 mmol),1,3-二羰基化合物(2 mmol),脲或硫脲(3 mmol),[C2O2BBTA][TFA] (20 mol%),90℃,40 min;b分离产率。
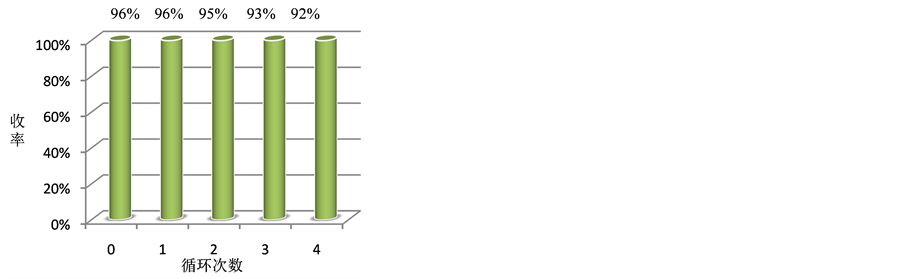
Figure 1. Recycling research of ionic liquid [C2O2BBTA][TFA]
图1. 离子液体[C2O2BBTA][TFA]的循环使用研究
4. 结论
本文发展了一种离子液体1-丁基-3-羧甲基苯并三唑三氟乙酸盐催化芳香醛、1,3-二羰基化合物和脲或硫脲绿色、高效合成3,4-二氢嘧啶-2(1H)-酮或硫酮的方法。该方法具有对环境友好、反应时间短、产率高等优点,离子液体催化剂可循环使用4次并且活性没有明显降低。
基金项目
国家自然科学基金(No. 21572195, 21262035, 21162025)。